Label: TIBETREE PAIN RELIEVEING MEDICATED PLASTER- camphor plaster
- NDC Code(s): 66506-186-01, 66506-186-02
- Packager: Tibet Cheezheng Tibetan Medicine Co. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- ASK DOCTOR
- Keep out of reach of children
- If pregnant
-
Directions
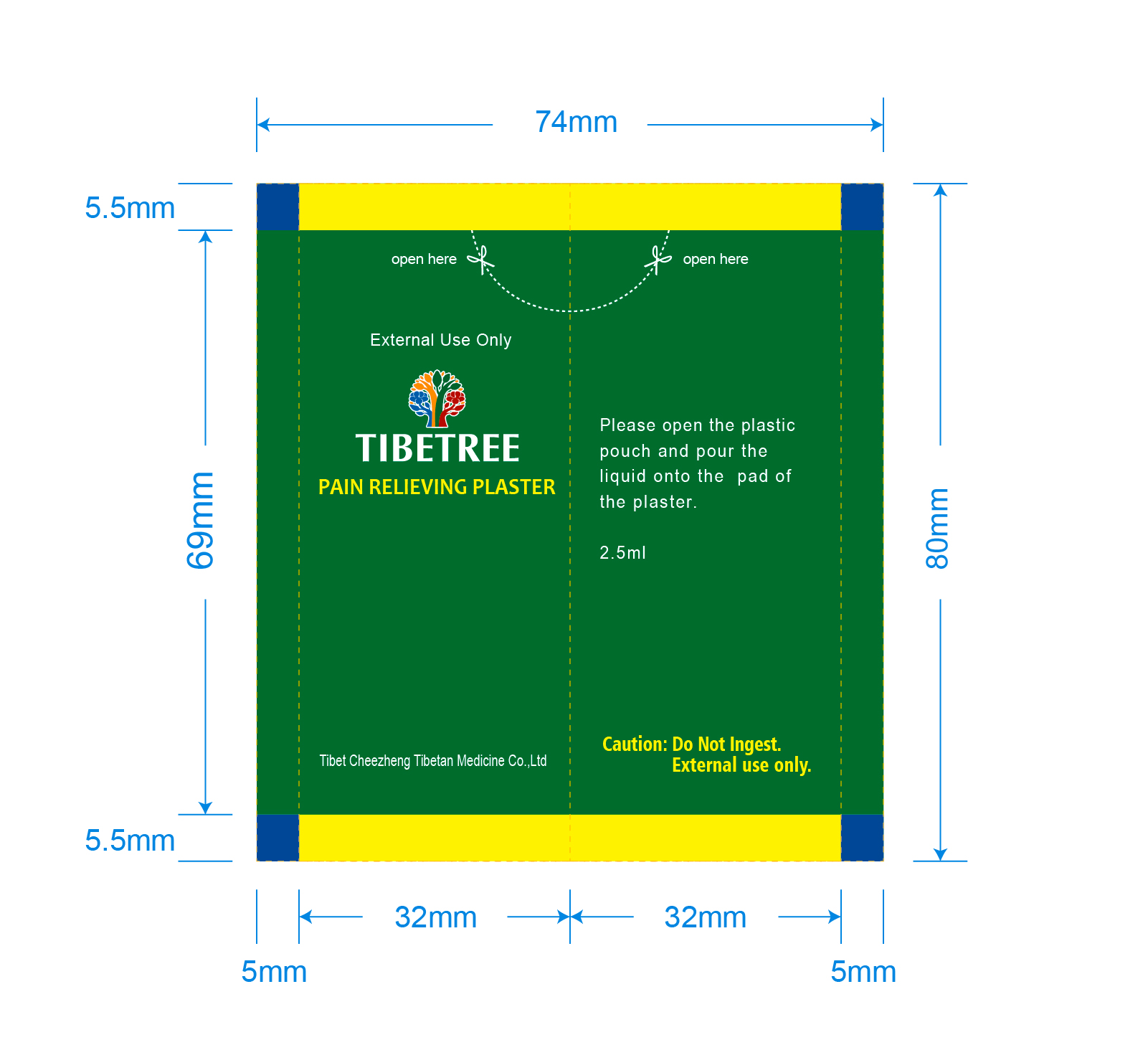
- Adults and children 12 years of age and older: remove protective film from the plaster, pour the liquid onto the pad of plaster and apply it to the painful area or acupoint as directed (see figure)
- Use 1 to 2 plasters every 24 hours
- Apply the plaster to the affected area for 4 to 8 hours.
- Do not apply to area with excessive hair. Adhesive plaster may hurt skin upon removal.
- Children under 12 years of age: Do not use or consult a doctor.
- Other information
- Inactive ingredients
- Questions?
- Purpose
- Do not use
- When using this product
- Stop use and ask a doctor if
- Uses
- Warnings
- Tibetree PDP
-
INGREDIENTS AND APPEARANCE
TIBETREE PAIN RELIEVEING MEDICATED PLASTER
camphor plasterProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66506-186 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 1 g in 100 g Inactive Ingredients Ingredient Name Strength ZANTHOXYLUM BUNGEANUM FRUIT RIND (UNII: K447936H97) CURCUMA LONGA WHOLE (UNII: W5488JUO8U) CARTHAMUS TINCTORIUS FLOWER BUD (UNII: B86IS274O0) PHLOMOIDES ROTATA WHOLE (UNII: 7CEU5N8573) MYRICARIA PANICULATA STEM (UNII: 4C36LPC9I2) OXYTROPIS CHILIOPHYLLA WHOLE (UNII: 9T7BW444B3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66506-186-01 5 in 1 PACKAGE 12/05/2019 1 NDC:66506-186-02 1.2 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/05/2019 Labeler - Tibet Cheezheng Tibetan Medicine Co. Ltd (529074851)