Label: CREST PRO-HEALTH- cetylpyridinium chloride rinse
- NDC Code(s): 37000-452-03, 37000-452-04
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
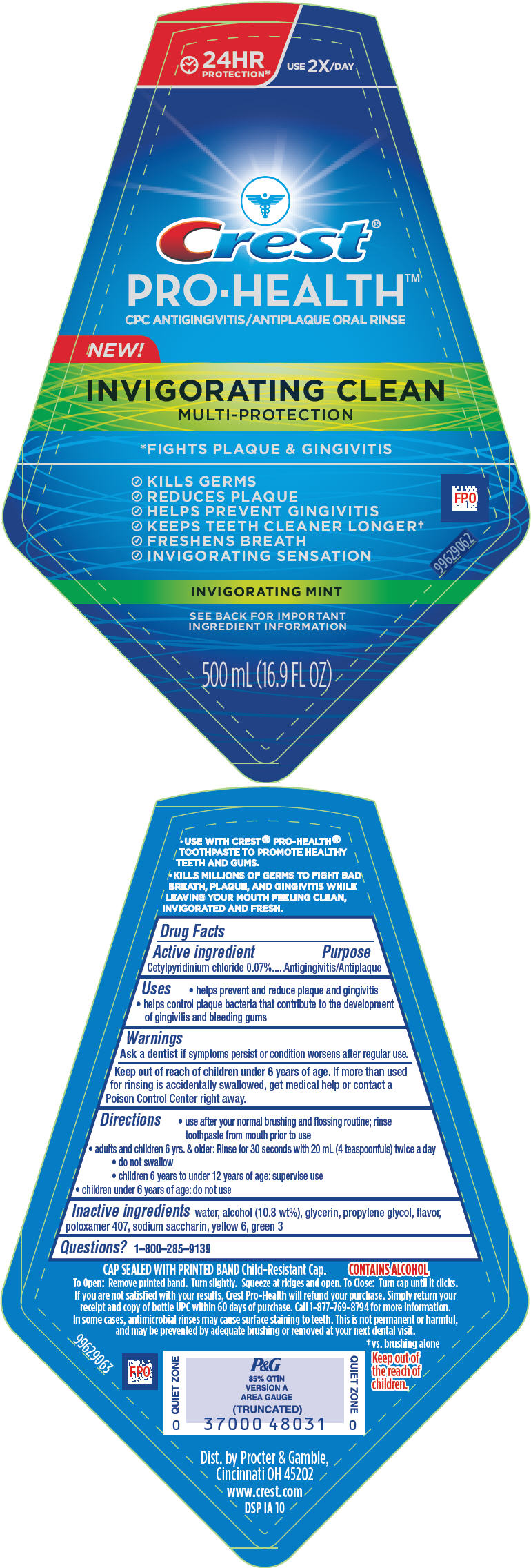
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- use after your normal brushing and flossing routine; rinse toothpaste from mouth prior to use
- adults and children 6 yrs. & older: Rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day
- do not swallow
- children 6 years to under 12 years of age: supervise use
- children under 6 years of age: do not use
- Inactive ingredients
- Questions?
-
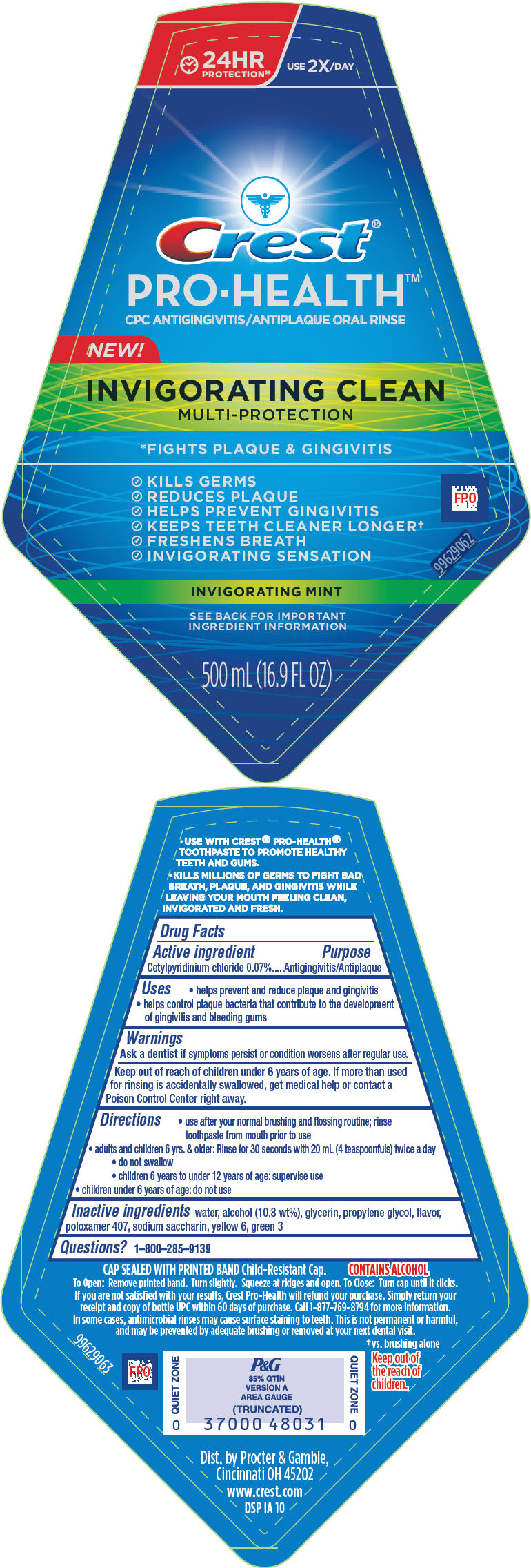
PRINCIPAL DISPLAY PANEL - 500 mL Bottle Label
24HR
PROTECTION*USE 2X/ DAY
Crest®
PRO-HEALTH™
CPC ANTIGINGIVITIS/ANTIPLAQUE ORAL RINSENEW!
INVIGORATING CLEAN
MULTI-PROTECTION*FIGHTS PLAQUE & GINGIVITIS
- KILLS GERMS
- REDUCES PLAQUE
- HELPS PREVENT GINGIVITIS
- KEEPS TEETH CLEANER LONGER†
- FRESHENS BREATH
- INVIGORATING SENSATION
FPO
INVIGORATING MINT
SEE BACK FOR IMPORTANT
INGREDIENT INFORMATION500 mL (16.9 FL OZ)
99629062
-
INGREDIENTS AND APPEARANCE
CREST PRO-HEALTH
cetylpyridinium chloride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37000-452 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.014 g in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLOXAMER 407 (UNII: TUF2IVW3M2) SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) Product Characteristics Color Score Shape Size Flavor WINTERGREEN Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37000-452-03 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/14/2011 09/10/2017 2 NDC:37000-452-04 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/14/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 03/14/2011 Labeler - The Procter & Gamble Manufacturing Company (004238200)