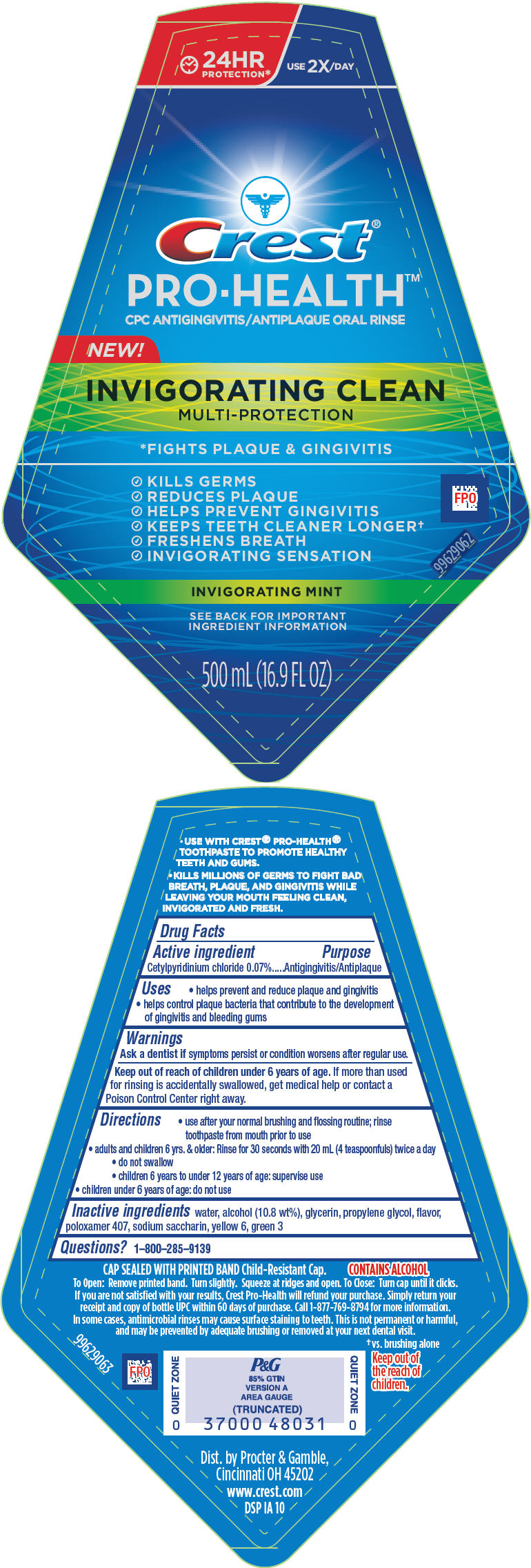
Uses
- helps prevent and reduce plaque and gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis and bleeding gums
Directions
- use after your normal brushing and flossing routine; rinse toothpaste from mouth prior to use
- adults and children 6 yrs. & older: Rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day
- do not swallow
- children 6 years to under 12 years of age: supervise use
- children under 6 years of age: do not use
Inactive ingredients
water, alcohol (10.8 wt%), glycerin, propylene glycol, flavor, poloxamer 407, sodium saccharin, yellow 6, green 3
PRINCIPAL DISPLAY PANEL - 500 mL Bottle Label
24HR
PROTECTION*
USE 2X/ DAY
Crest®
PRO-HEALTH™
CPC ANTIGINGIVITIS/ANTIPLAQUE ORAL RINSE
NEW!
INVIGORATING CLEAN
MULTI-PROTECTION
*FIGHTS PLAQUE & GINGIVITIS
- KILLS GERMS
- REDUCES PLAQUE
- HELPS PREVENT GINGIVITIS
- KEEPS TEETH CLEANER LONGER†
- FRESHENS BREATH
- INVIGORATING SENSATION
FPO
INVIGORATING MINT
SEE BACK FOR IMPORTANT
INGREDIENT INFORMATION
500 mL (16.9 FL OZ)
99629062