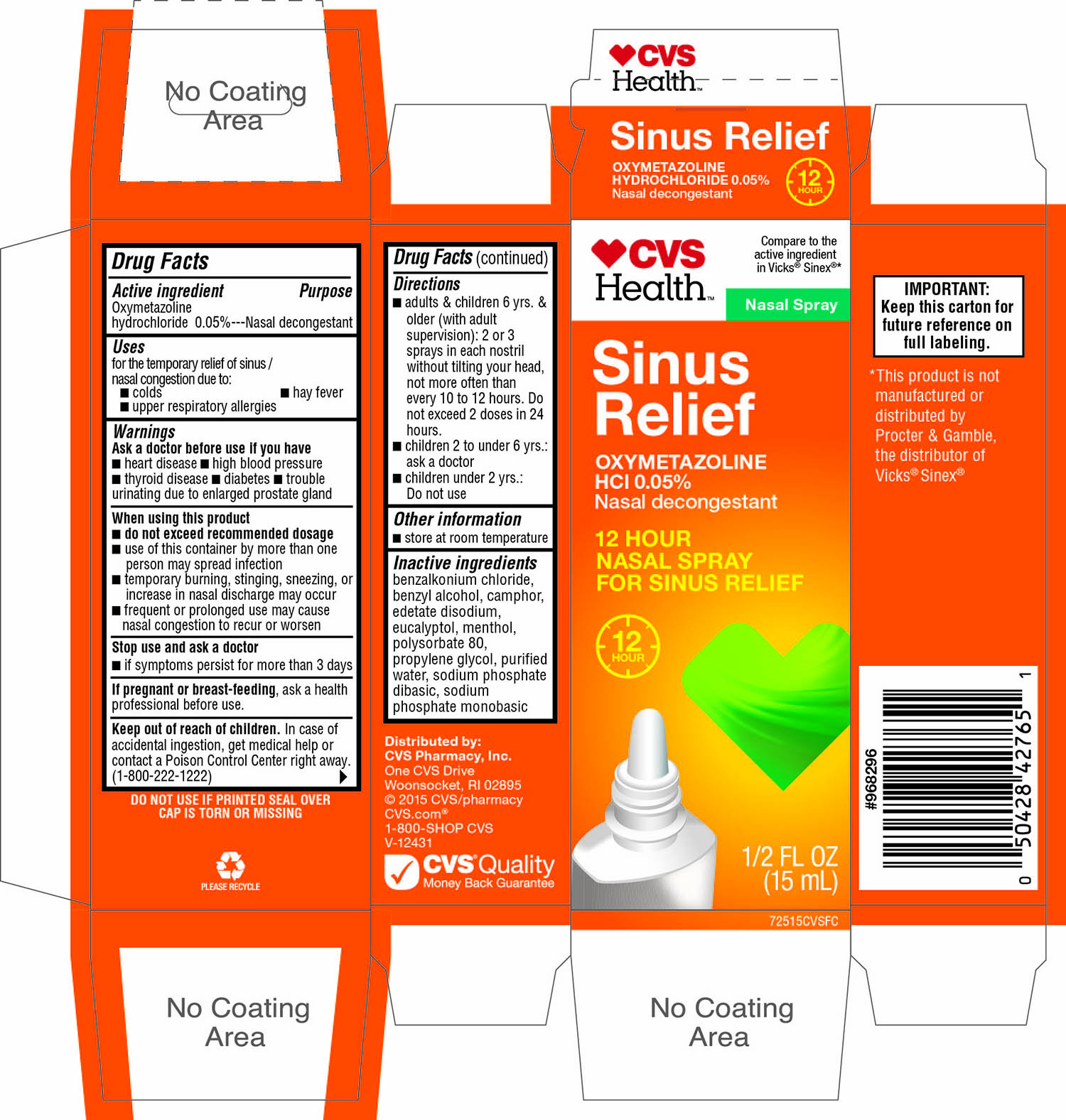
Label: CVS HEALTH SINUS RELIEF- oxymetazoline hydrochloride spray
- NDC Code(s): 59779-787-15
- Packager: CVS Pharmacy,Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 2, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to enlarged prostate gland
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel
CVS Health ™
Compare to the active ingredient in Vicks® Sinex® *
Nasal Spray
Sinus Relief
OXYMETAZOLINE HCl 0.05%
Nasal decongestant
12 HOUR
NASAL SPRAY FOR SINUS RELIEF
12 Hour
1/2 FL OZ (15 mL)
DO NOT USE IF PRINTED SEAL OVER CAP IS TORN OR MISSNG
IMPORTANT: Keep this carton for future reference on full labeling.
*This product is not manufactured or distributed by Procter & Gamble, the distributor of Vicks®Sinex®
Distributed by:
CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
© 2015 CVS/pharmacy
CVS.com®
1-800-SHOP CVS
V-12431
CVS Quality
Money Back Guarantee
-
INGREDIENTS AND APPEARANCE
CVS HEALTH SINUS RELIEF
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-787 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.00050 g in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) CAMPHOR (NATURAL) (UNII: N20HL7Q941) EDETATE DISODIUM (UNII: 7FLD91C86K) EUCALYPTOL (UNII: RV6J6604TK) MENTHOL (UNII: L7T10EIP3A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-787-15 1 in 1 CARTON 04/21/2015 1 15 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 04/21/2015 Labeler - CVS Pharmacy,Inc. (062312574)