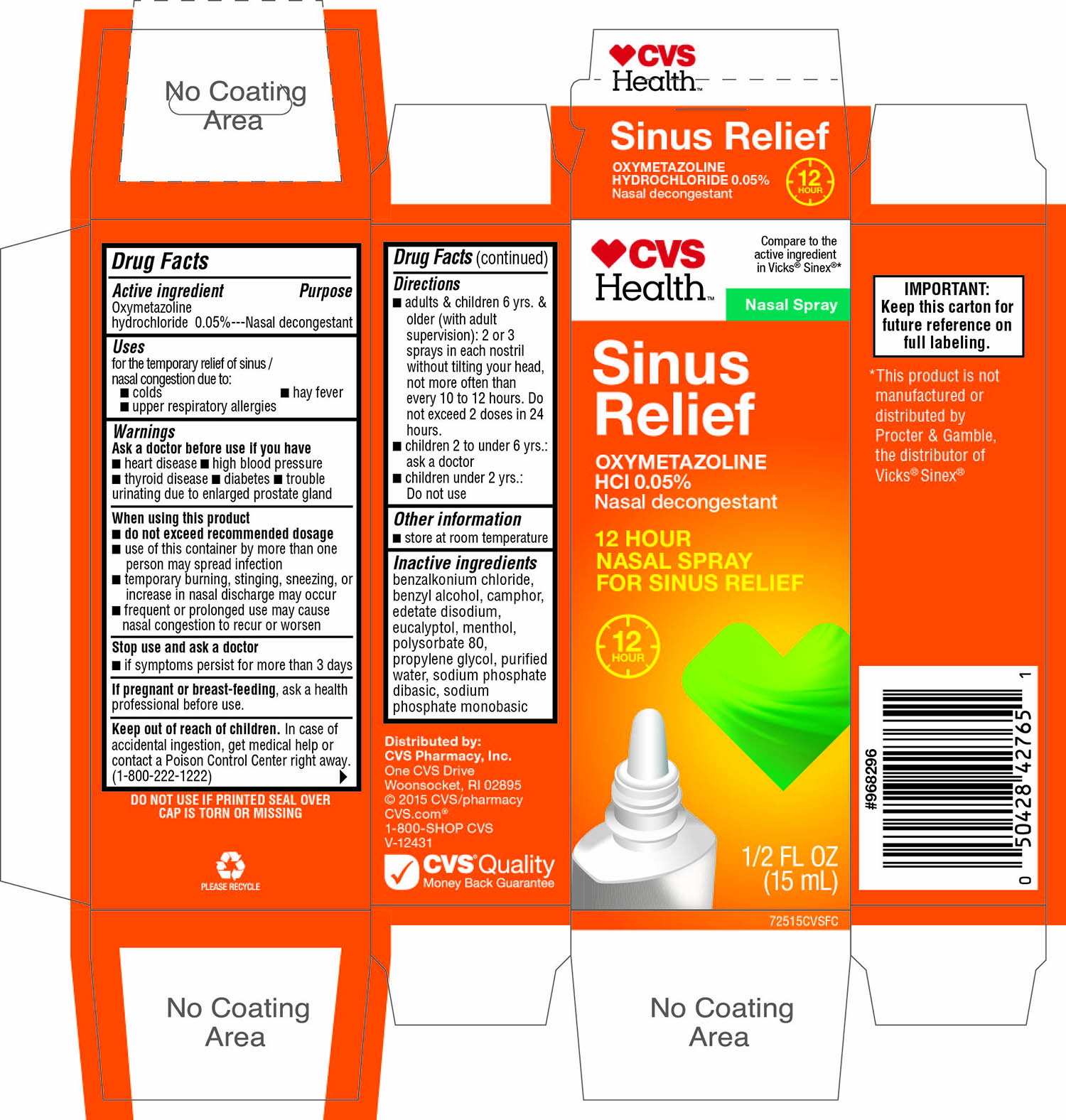
Uses
- •
- for the temporary relief of sinus/ nasal congestion due to:
- •
- colds
- •
- hay fever
- •
- upper respiratory allergies
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to enlarged prostate gland
Directions
- •
- adults and children 6 yrs, & older (with adult supervision): 2 or 3 sprays in each nostril without tilting your head, not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24 hours.
- •
- children 2 to under 6 yrs : ask a doctor
- •
- children under 2 yrs: Do not use
Inactive ingredients
benzalkonium chloride, benzyl alcohol, camphor, edetate disodium, eucalyptol, menthol, polysorbate 80,propylene glycol, purified water, sodium phosphate dibasic, sodium phosphate monobasic
Principal Display Panel
CVS Health ™
Compare to the active ingredient in Vicks® Sinex® *
Nasal Spray
Sinus Relief
OXYMETAZOLINE HCl 0.05%
Nasal decongestant
12 HOUR
NASAL SPRAY FOR SINUS RELIEF
12 Hour
1/2 FL OZ (15 mL)
DO NOT USE IF PRINTED SEAL OVER CAP IS TORN OR MISSNG
IMPORTANT: Keep this carton for future reference on full labeling.
*This product is not manufactured or distributed by Procter & Gamble, the distributor of Vicks®Sinex®
Distributed by:
CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
© 2015 CVS/pharmacy
CVS.com®
1-800-SHOP CVS
V-12431
CVS Quality
Money Back Guarantee