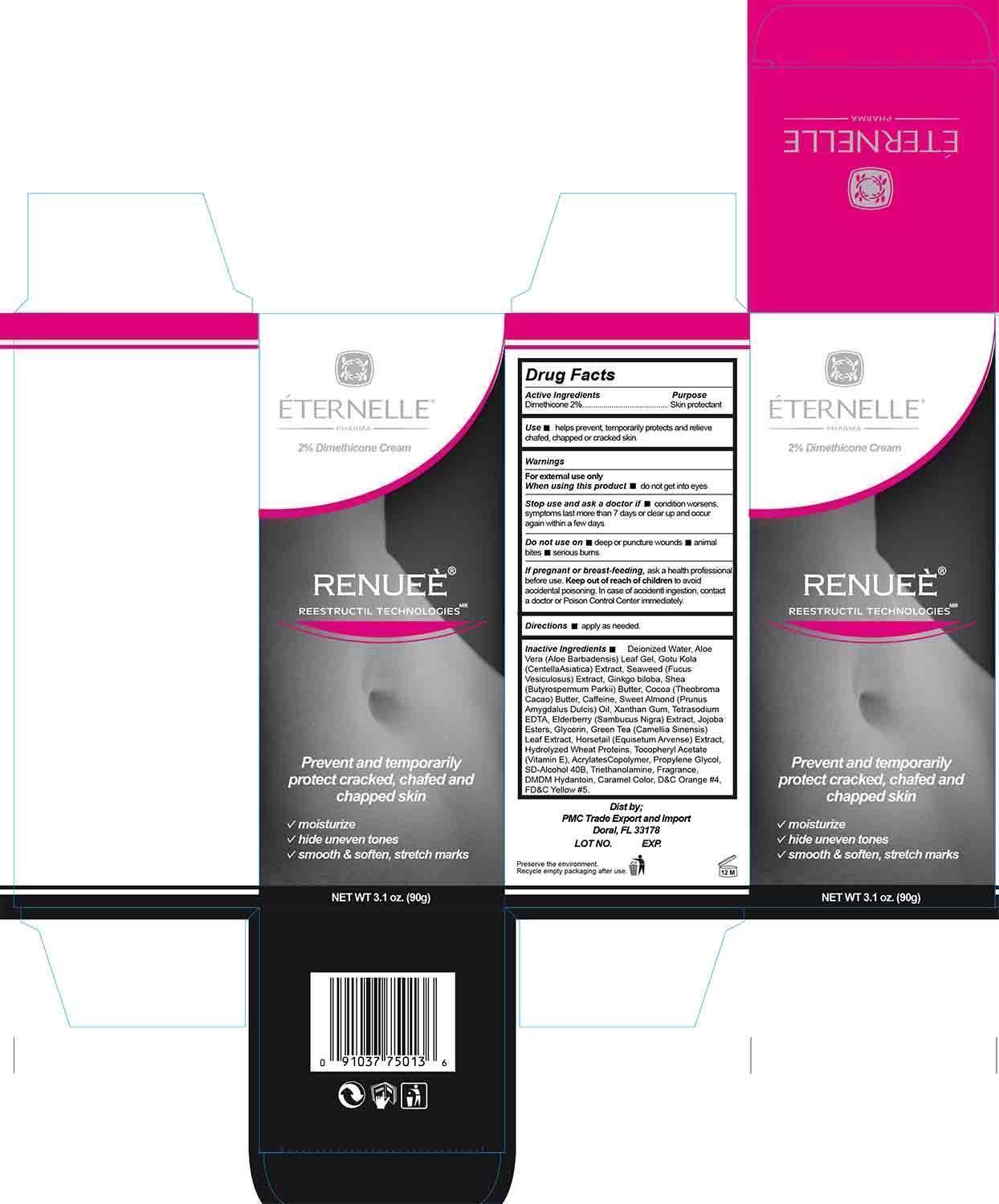
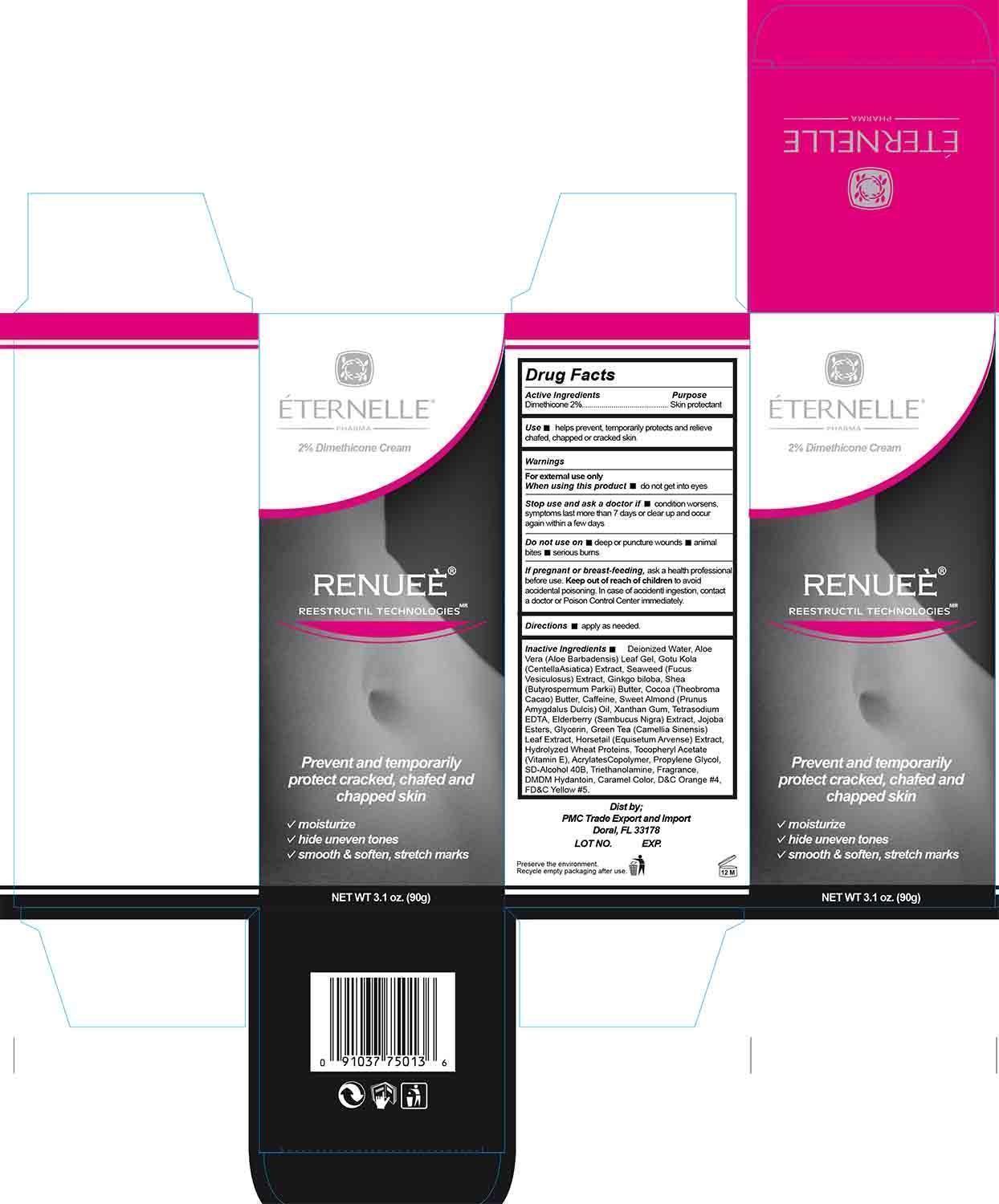
Label: RENUEE- dimethicone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 68973-002-90 - Packager: PMC TRADE, EXPORT AND IMPORT
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 29, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- When using this product
- Stop use and ask a doctor if
- Do not use on
- If pregnant or breast feeding
- Keep out of reach of children
- Directions
-
Inactive Ingredients
Deionized Water, Aloe Vera (Aloe Barbadensis) Leaf Gel, Gotu Kola (Centella Asiatica) Extract, Seaweed (Fucus Vesiculosus) Extract, Ginkgo biloba, Shea (Butyrospermum Parkii) Butter, Cocoa (Theobroma Cacao) Butter, Caffeine, Sweet Almond (Prunus Amygdalus Dulcis) Oil, Xanthan Gum, Tetrasodium EDTA, Elderberry (Sambucus Nigra) Extract, Jojoba Esters, Glycerin, Green Tea (Camellia Sinensis) Leaf Extract, Horsetail (Equisetum Arvense) Extract, Hydrolyzed Wheat Proteins, Tocopheryl Acetate (Vitamin E), Acrylates Copolymer, Propylene Glycol, SD-Alcohol 40B, Triethanolamine, Fragrance, DMDM Hydantoin, Caramel Color, D&C Orange #4, FD&C Yellow #5
- Product Label
-
INGREDIENTS AND APPEARANCE
RENUEE
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68973-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) CENTELLA ASIATICA (UNII: 7M867G6T1U) FUCUS VESICULOSUS (UNII: 535G2ABX9M) GINKGO BILOBA WHOLE (UNII: 660486U6OI) SHEA BUTTER (UNII: K49155WL9Y) CAFFEINE (UNII: 3G6A5W338E) ALMOND OIL (UNII: 66YXD4DKO9) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE SODIUM (UNII: MP1J8420LU) SAMBUCUS NIGRA FLOWERING TOP (UNII: CT03BSA18U) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) GLYCERIN (UNII: PDC6A3C0OX) GREEN TEA LEAF (UNII: W2ZU1RY8B0) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HYDROLYZED WHEAT PROTEIN (ENZYMATIC, 3000 MW) (UNII: J2S07SB0YL) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) DMDM HYDANTOIN (UNII: BYR0546TOW) D&C ORANGE NO. 11 (UNII: B16H36W4W4) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68973-002-90 90 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 07/15/2014 Labeler - PMC TRADE, EXPORT AND IMPORT (179635003)