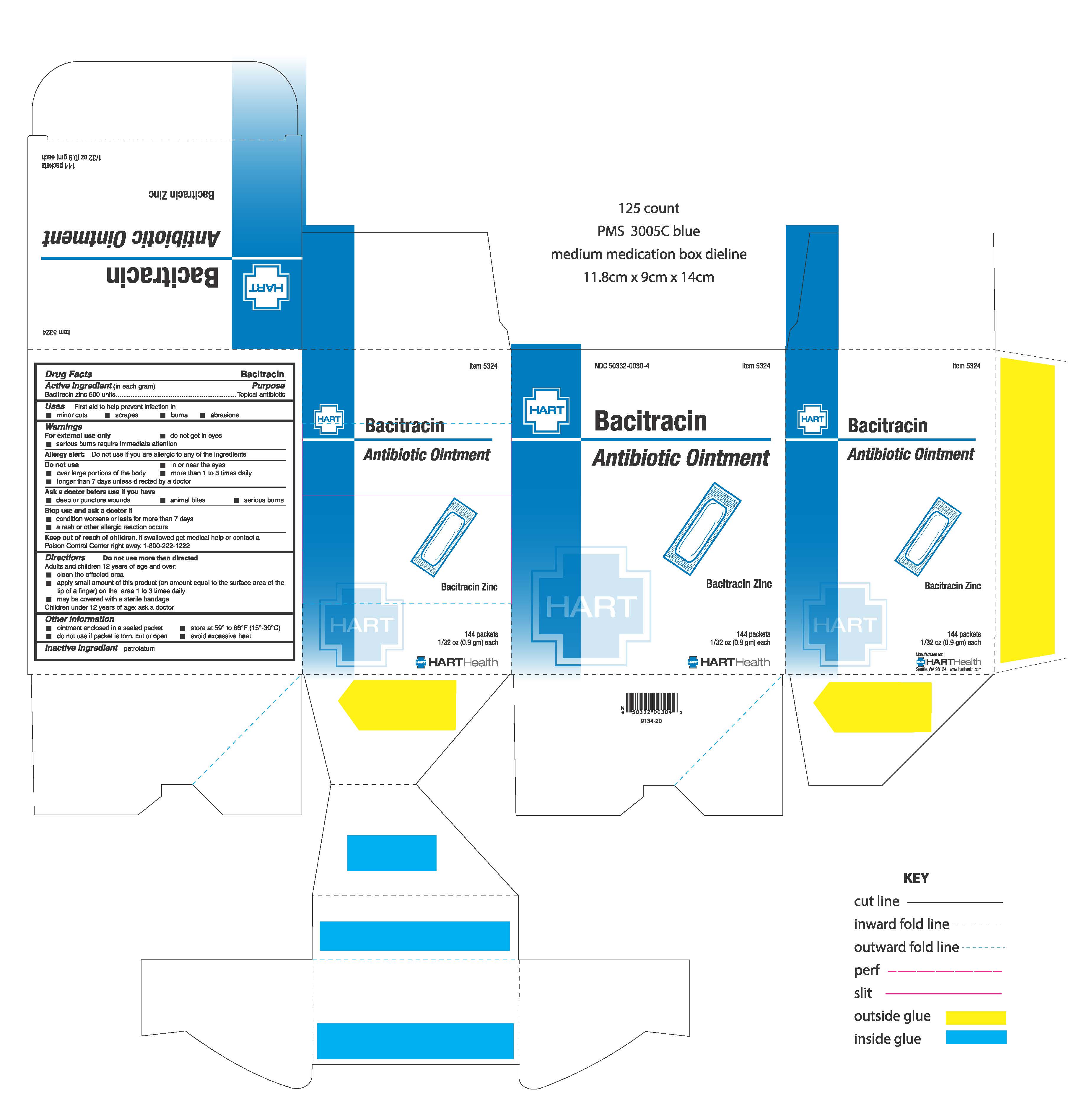
Label: BACITRACIN- bacitracin zinc cream
- NDC Code(s): 50332-0030-4
- Packager: HART Health
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 24, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions: do not use more than directed
Adults and children 12 years of age and over:
- clean the affected area
- apply small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Children under 12 years of age: ask a doctor
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACITRACIN
bacitracin zinc creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50332-0030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 500 U in 0.9 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50332-0030-4 144 in 1 BOX, UNIT-DOSE 01/05/2021 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 01/05/2021 Labeler - HART Health (069560969) Registrant - HART Health (069560969) Establishment Name Address ID/FEI Business Operations WaterJel Technologies 155522589 manufacture(50332-0030)