Label: TENDERWRAP- zinc oxide, calamine dressing
-
Contains inactivated NDC Code(s)
NDC Code(s): 12823-401-35, 12823-401-36 - Packager: Tyco Healthcare Group LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 29, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warning
-
Directions
- Begin bandaging at base of toes, without pressure, keeping foot and leg at right angle.
- Continue bandaging beyond ankle, doubling back to ensure molding to contours of the leg.
- Complete bandaging to just below the knee and apply an adhesive elastic bandage to secure the Unna Boot.
- Re-apply as needed
- Begin bandaging at base of toes, without pressure, keeping foot and leg at right angle.
- Other Ingredients
-
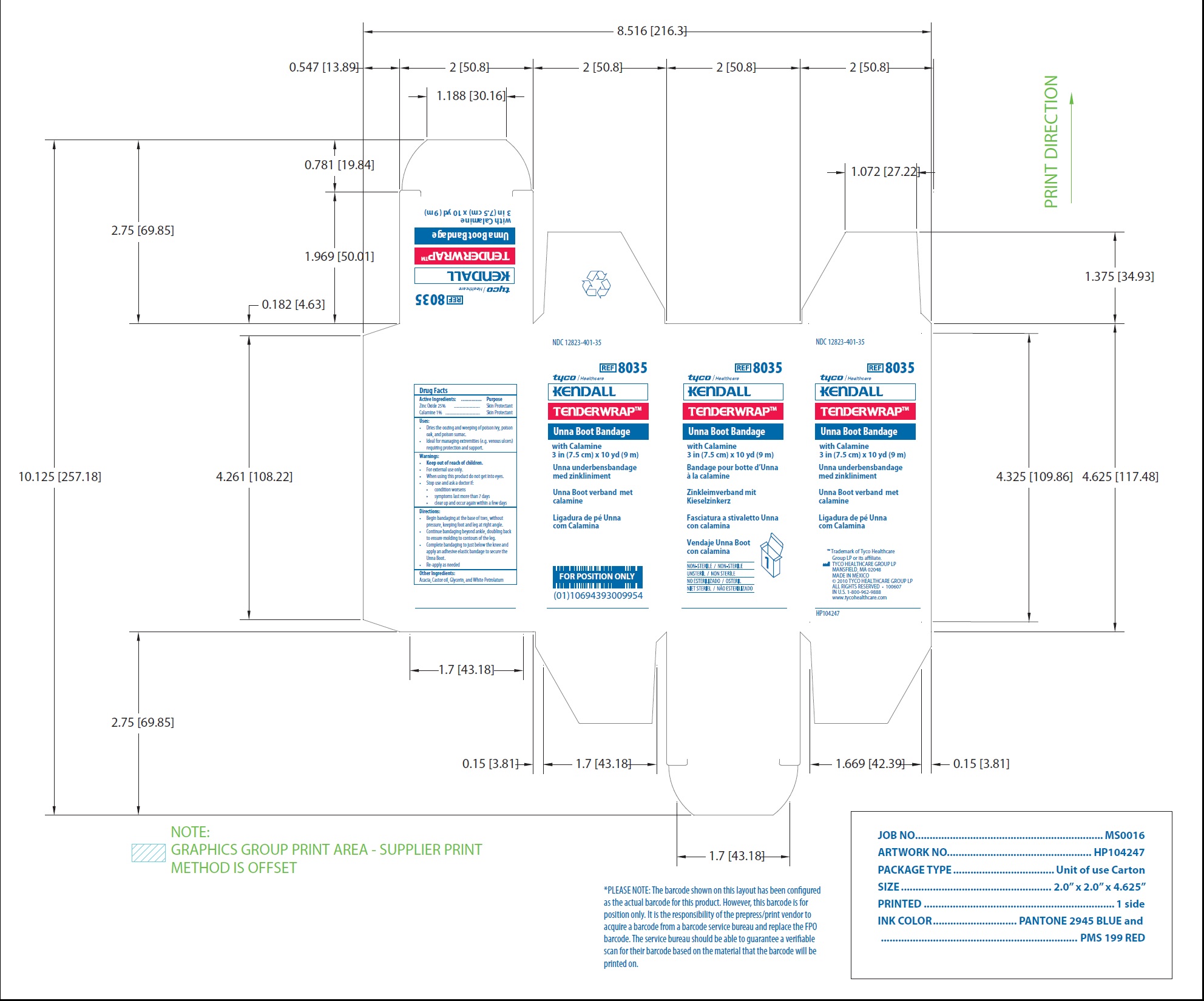
Principal Display Panel
NDC 12823-401-35
REF 8035
tyco/Healthcare
KENDALL
TENDERWRAP TM
Unna Boot Bandage
with Calamine
3 in (7.5 cm) x 10 yd (9 m)
Unna underbensbandage
med zinkliniment
Unna Boot verband met
calamine
Ligadura de pe Unna
com Calamina
Bandage pour botte d'Unna
a la calamine
Zinkleimverband mit
kieselzinkerz
Fasciatura a stivaletto Unna
con calamina
Vendaje Unna Boot
con calamina
NON-STERILE / NON-STERILE
UNSTERIL / NON STERILE
NO ESTERILIZADO/ OSTERIL
NIET STERIEL / NAO ESTERILIZADO
TM Trademark of Tyco Healthcare Group LP or its affiliate.
TYCO HEALTHCARE GROUP LP
MANSFIELD, MA 02048
MADE IN MEXICO
2010 TYCO HEALTHCARE GROUP LP
ALL RIGHTS RESERVED 100607
in U.S. 1-800-962-9888
www.tycohealthcare.com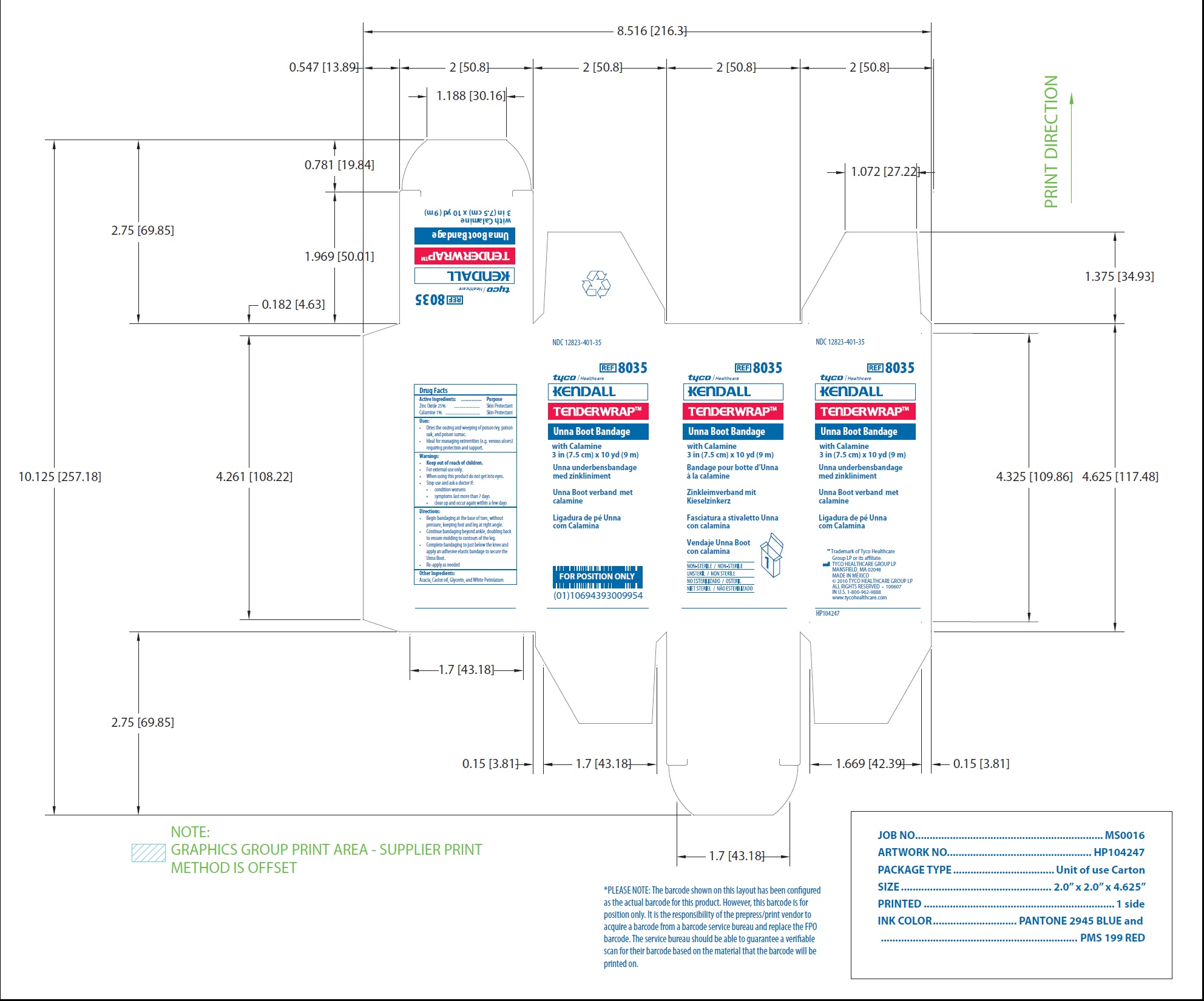
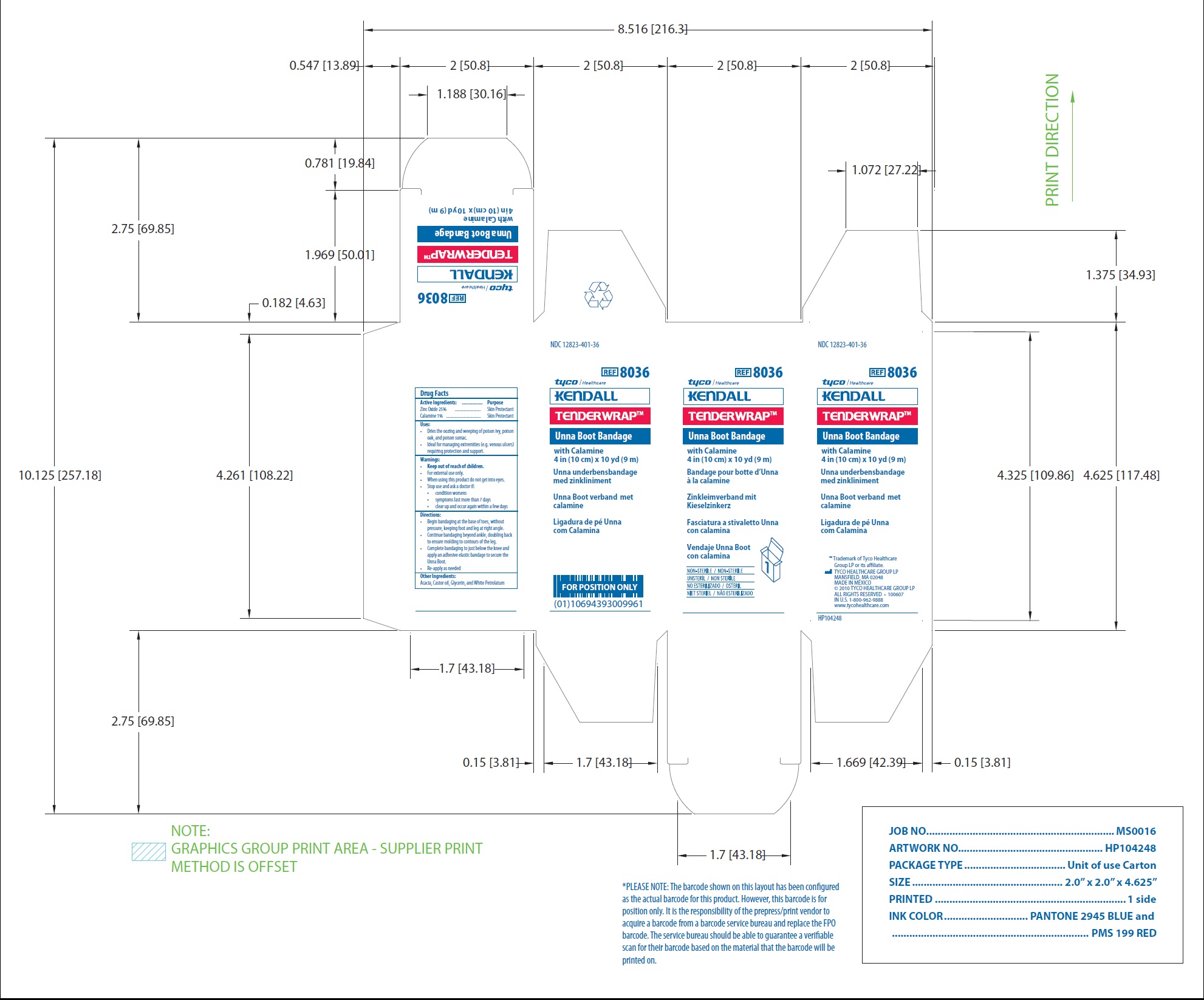
NDC 12823-401-36
REF 8036
tyco/Healthcare
KENDALL
TENDERWRAP TM
Unna Boot Bandage
with Calamine
4 in (10 cm) x 10 yd (9 m)
Unna underbensbandage
med zinkliniment
Unna Boot verband met
calamine
Ligadura de pe Unna
com Calamina
Bandage pour botte d'Unna
a la calamine
Zinkleimverband mit
kieselzinkerz
Fasciatura a stivaletto Unna
con calamina
Vendaje Unna Boot
con calamina
NON-STERILE / NON-STERILE
UNSTERIL / NON STERILE
NO ESTERILIZADO/ OSTERIL
NIET STERIEL / NAO ESTERILIZADO
TM Trademark of Tyco Healthcare Group LP or its affiliate.
TYCO HEALTHCARE GROUP LP
MANSFIELD, MA 02048
MADE IN MEXICO
2010 TYCO HEALTHCARE GROUP LP
ALL RIGHTS RESERVED 100607
in U.S. 1-800-962-9888
www.tycohealthcare.com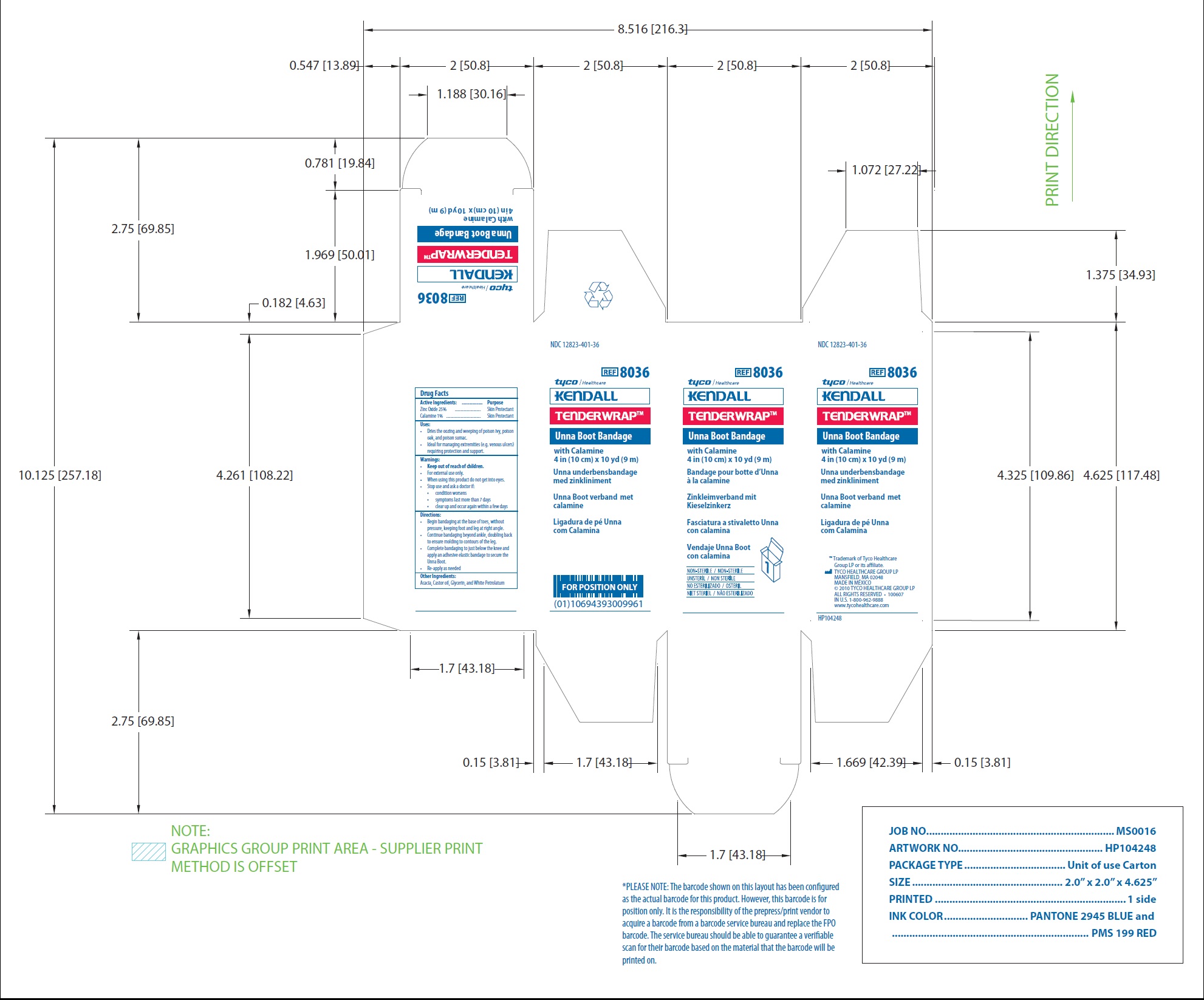
-
INGREDIENTS AND APPEARANCE
TENDERWRAP
zinc oxide, calamine dressingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:12823-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC OXIDE 25 g FERRIC OXIDE RED (UNII: 1K09F3G675) (FERRIC OXIDE RED - UNII:1K09F3G675) FERRIC OXIDE RED 0.015 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC OXIDE 0.985 g Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) CASTOR OIL (UNII: D5340Y2I9G) GLYCERIN (UNII: PDC6A3C0OX) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12823-401-36 1 in 1 BOX 2 NDC:12823-401-35 1 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 07/29/2010 Labeler - Tyco Healthcare Group LP (048696421)