Uses
- Dries the oozing and weeping of poison ivy, poison oak, or poison sumac.
- Ideal for the managing extremities (e.g. varicose ulcers) requiring protection and support.
Warning
- Keep out of reach of children.
- For external use only.
- When using this product do not get into eyes.
- Stop use and ask a doctor if:
● symptoms last more than 7 days
● clear up and occur again within a few days.
Directions
- Begin bandaging at base of toes, without pressure, keeping foot and leg at right angle.
- Continue bandaging beyond ankle, doubling back to ensure molding to contours of the leg.
- Complete bandaging to just below the knee and apply an adhesive elastic bandage to secure the Unna Boot.
- Re-apply as needed
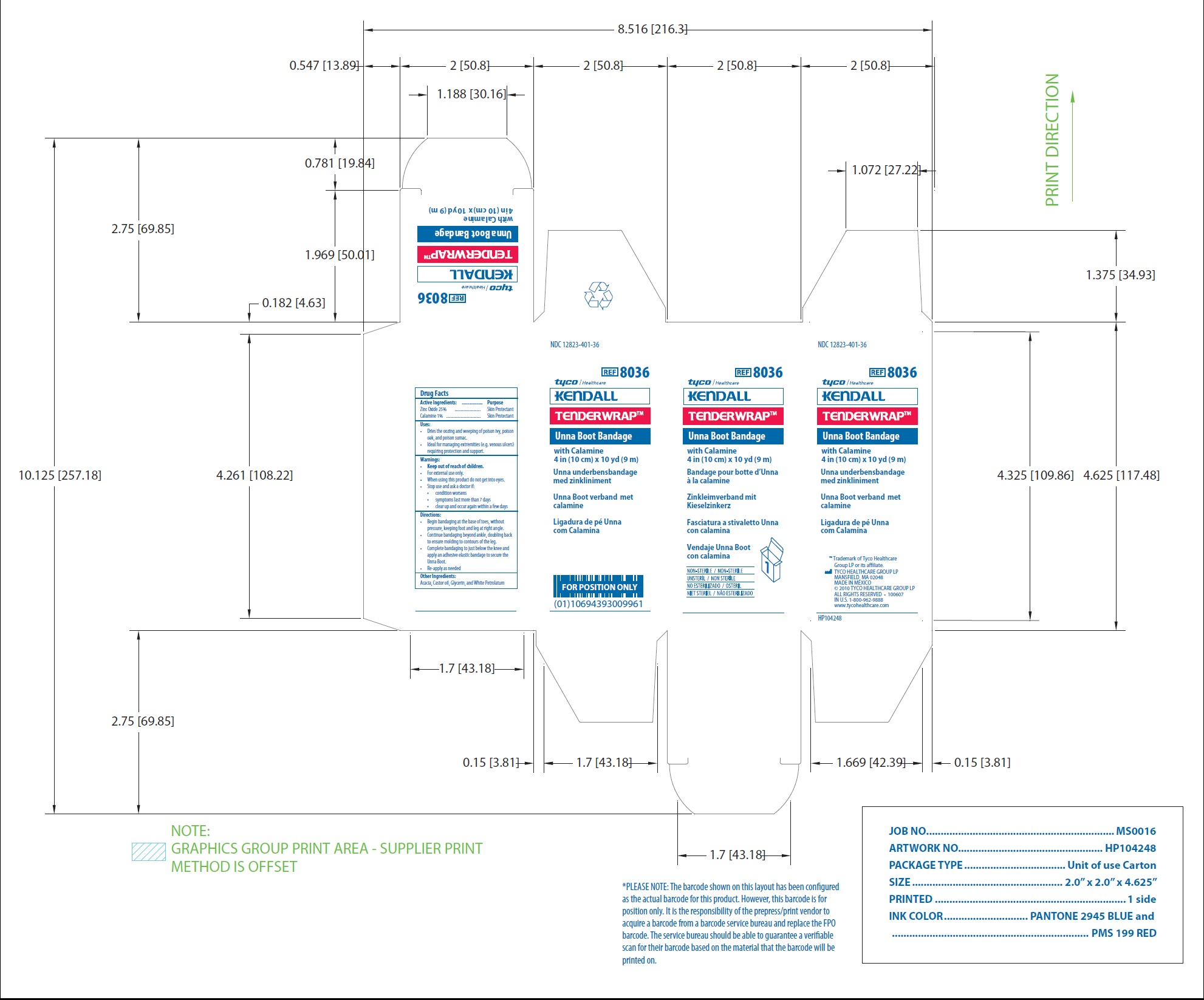
Principal Display Panel
NDC 12823-401-35
REF 8035
tyco/Healthcare
KENDALL
TENDERWRAP TM
Unna Boot Bandage
with Calamine
3 in (7.5 cm) x 10 yd (9 m)
Unna underbensbandage
med zinkliniment
Unna Boot verband met
calamine
Ligadura de pe Unna
com Calamina
Bandage pour botte d'Unna
a la calamine
Zinkleimverband mit
kieselzinkerz
Fasciatura a stivaletto Unna
con calamina
Vendaje Unna Boot
con calamina
NON-STERILE / NON-STERILE
UNSTERIL / NON STERILE
NO ESTERILIZADO/ OSTERIL
NIET STERIEL / NAO ESTERILIZADO
TM Trademark of Tyco Healthcare Group LP or its affiliate.
TYCO HEALTHCARE GROUP LP
MANSFIELD, MA 02048
MADE IN MEXICO
2010 TYCO HEALTHCARE GROUP LP
ALL RIGHTS RESERVED 100607
in U.S. 1-800-962-9888
www.tycohealthcare.com
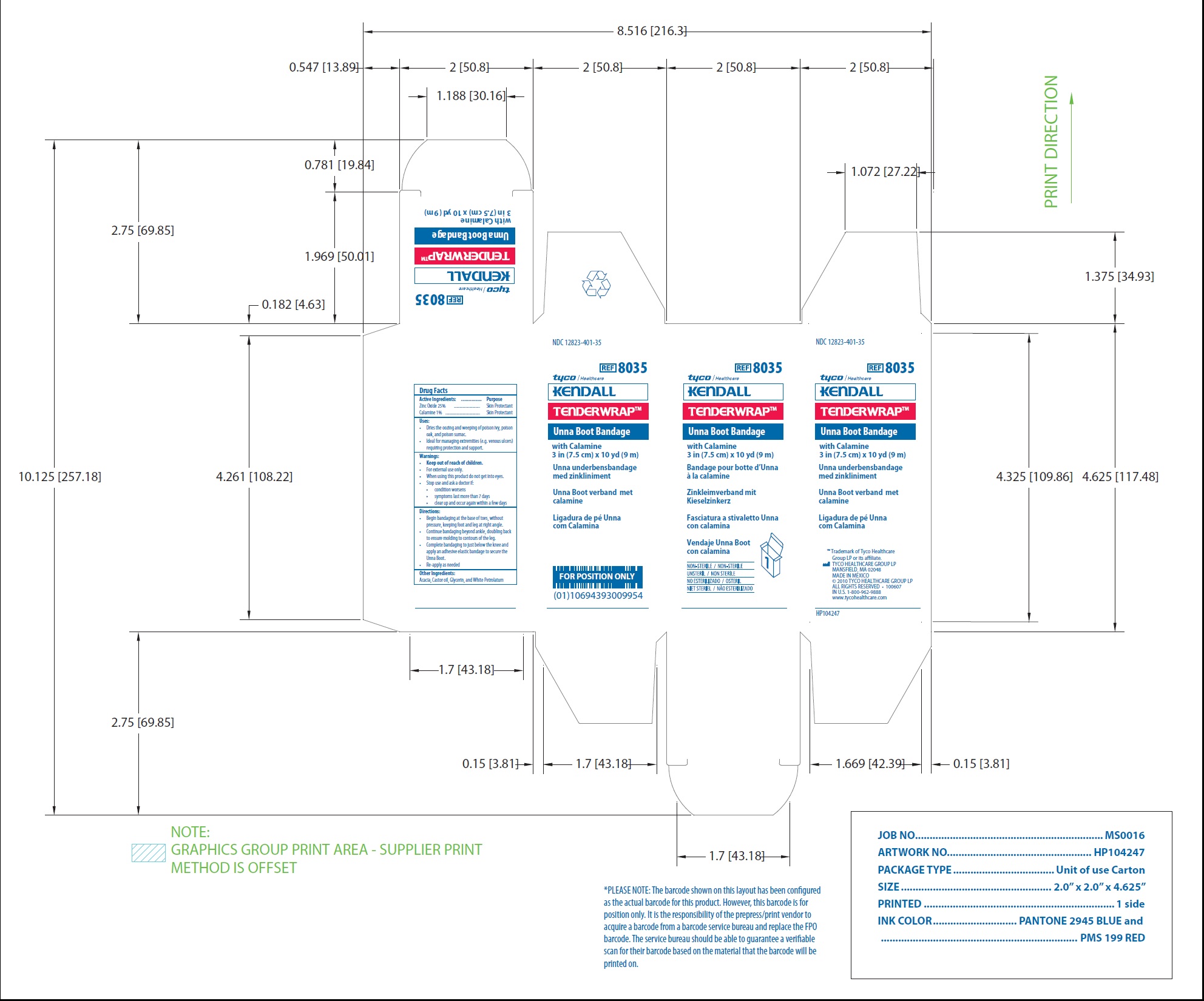
NDC 12823-401-36
REF 8036
tyco/Healthcare
KENDALL
TENDERWRAP TM
Unna Boot Bandage
with Calamine
4 in (10 cm) x 10 yd (9 m)
Unna underbensbandage
med zinkliniment
Unna Boot verband met
calamine
Ligadura de pe Unna
com Calamina
Bandage pour botte d'Unna
a la calamine
Zinkleimverband mit
kieselzinkerz
Fasciatura a stivaletto Unna
con calamina
Vendaje Unna Boot
con calamina
NON-STERILE / NON-STERILE
UNSTERIL / NON STERILE
NO ESTERILIZADO/ OSTERIL
NIET STERIEL / NAO ESTERILIZADO
TM Trademark of Tyco Healthcare Group LP or its affiliate.
TYCO HEALTHCARE GROUP LP
MANSFIELD, MA 02048
MADE IN MEXICO
2010 TYCO HEALTHCARE GROUP LP
ALL RIGHTS RESERVED 100607
in U.S. 1-800-962-9888
www.tycohealthcare.com