Label: EAR WAX REMOVAL AID DROPS- carbamide peroxide liquid
- NDC Code(s): 21130-793-15
- Packager: BETTER LIVING BRANDS, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
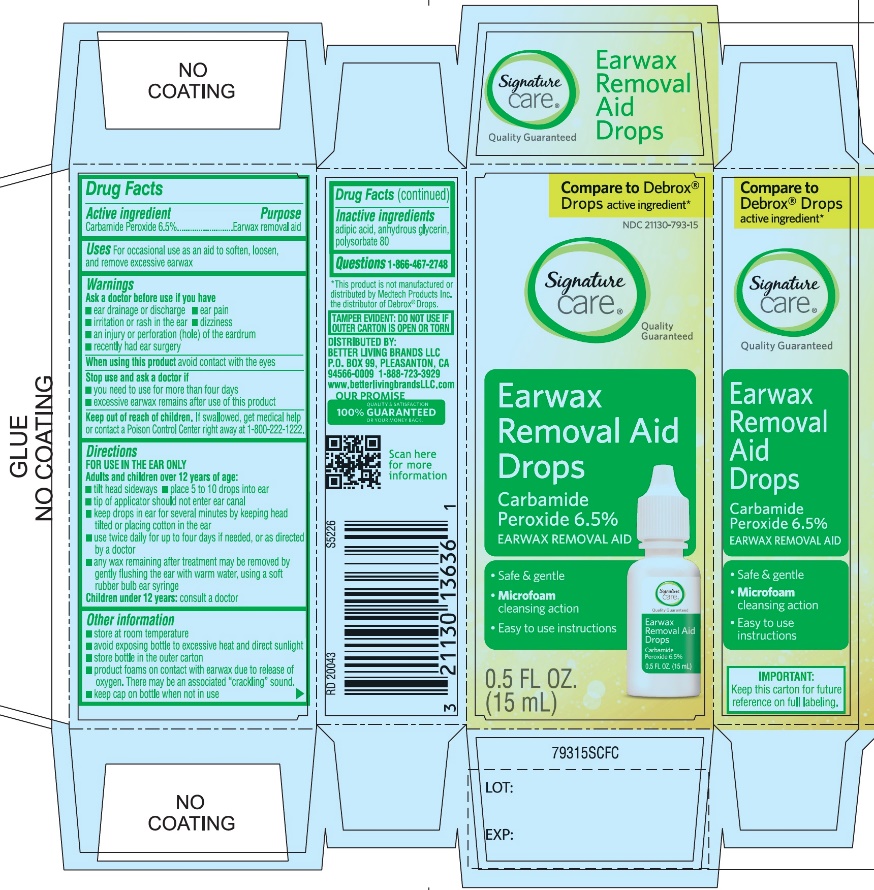
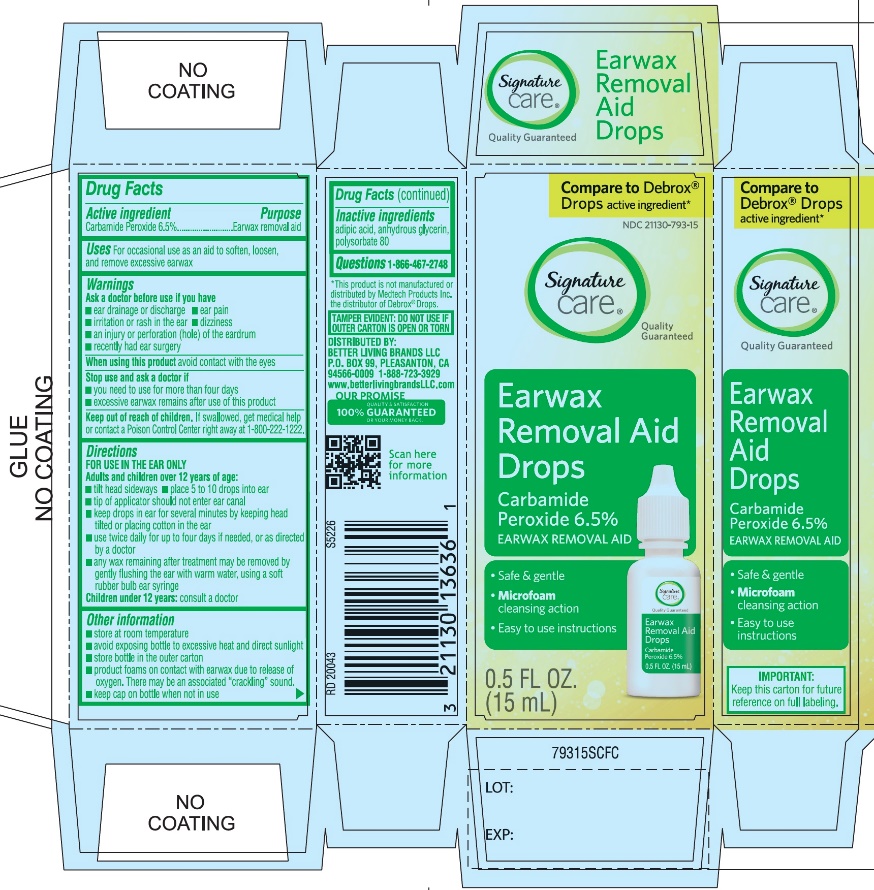
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use
- ▪
- if you have ear drainage or discharge
- ▪
- ear pain
- ▪
- irritation or rash in the ear
- ▪
- dizziness
- ▪
- an injury or perforation (hole) of the eardrum
- ▪
- recently had ear surgery
-
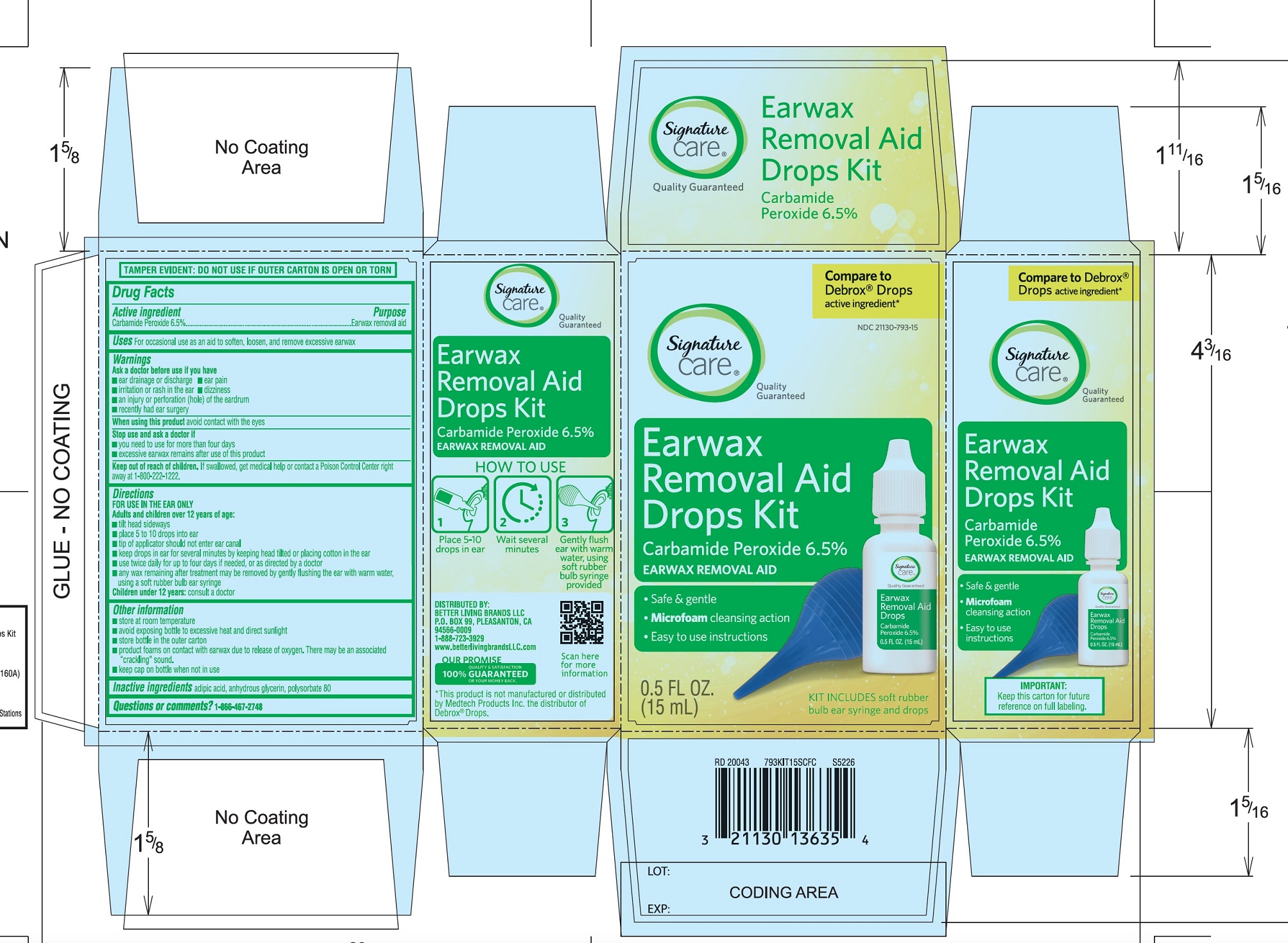
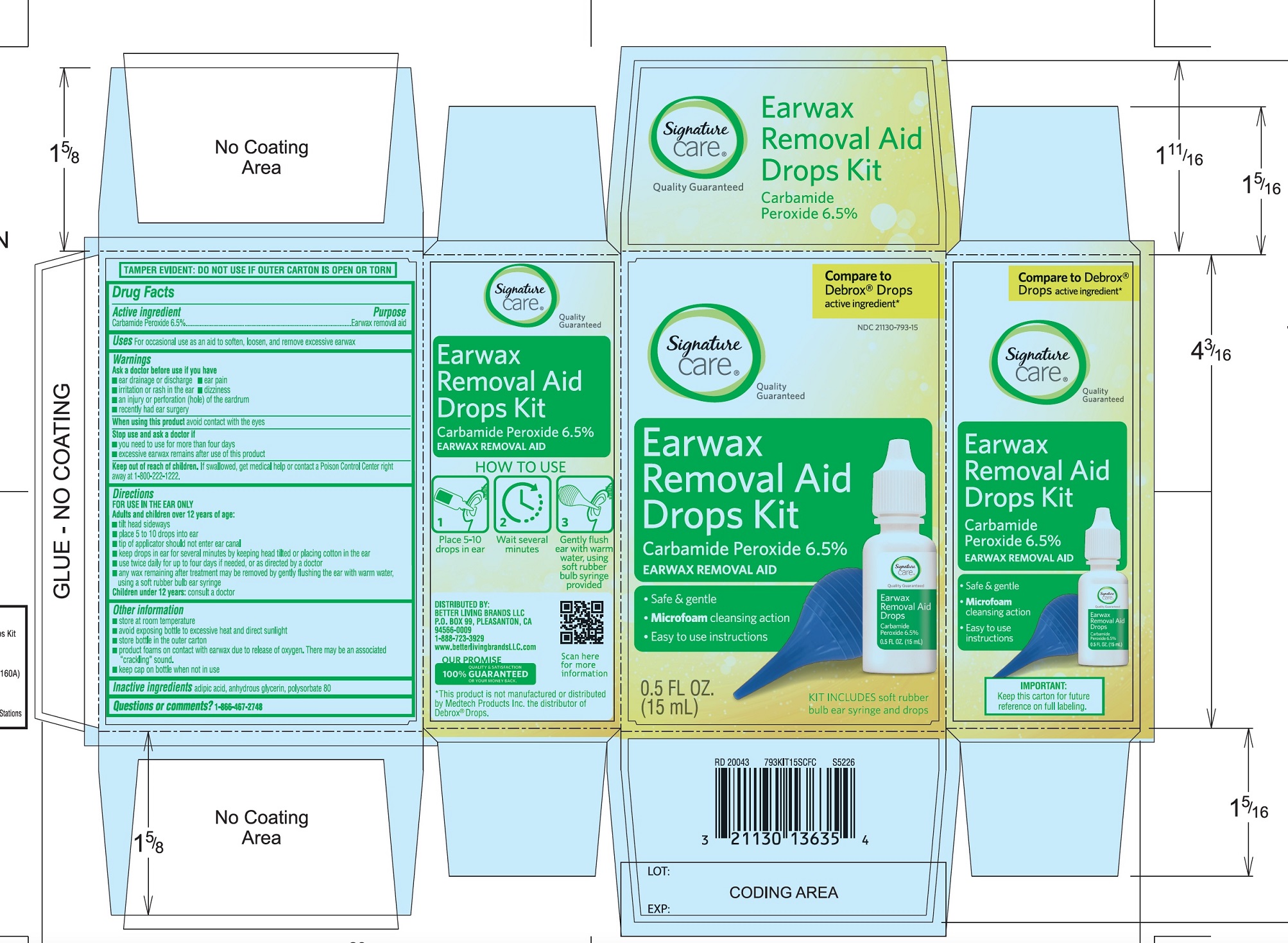
Directions
FOR USE IN THE EAR ONLY.
- ▪
- Adults and children over 12 years of age:
- ▪
- tilt head sideways
- ▪
- place 5 to 10 drops into ear canal.
- ▪
- tip of applicator should not enter ear canal.
- ▪
- keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear.
- ▪
- use twice daily for up to 4 days if needed, or as directed by a doctor.
- ▪
- any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe.
Children under 12 years of age: consult a doctor.
- Other information
- Inactive ingredients
- Questions? 1-866-467-2748
-
PRINCIPAL DISPLAY PANEL
*Compare to the active ingredient in Debrox® Drops
NDC 21130-793-15
Ear Wax Removal Aid Drops
Carbamide Peroxide 6.5%Ear Wax Removal Aid
- •
- Safe & Gentle
- •
- Microfoam Cleansing Action
- •
- Easy to use Instructions
NET WT 0.5 FL OZ (15 mL)
TAMPER EVIDENT: DO NOT USE IF OUTER CARTON IS OPEN OR TORN
DISTRIBUTED BY BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
1-888-723-3929
IMPORTANT: Keep this carton for future labeling.
*This product is not manufactured or distributed by Medtech Products Inc. the distributor of Debrox® Drops.
-
INGREDIENTS AND APPEARANCE
EAR WAX REMOVAL AID DROPS
carbamide peroxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-793 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-793-15 1 in 1 CARTON 01/13/2020 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/13/2020 Labeler - BETTER LIVING BRANDS, LLC (009137209)