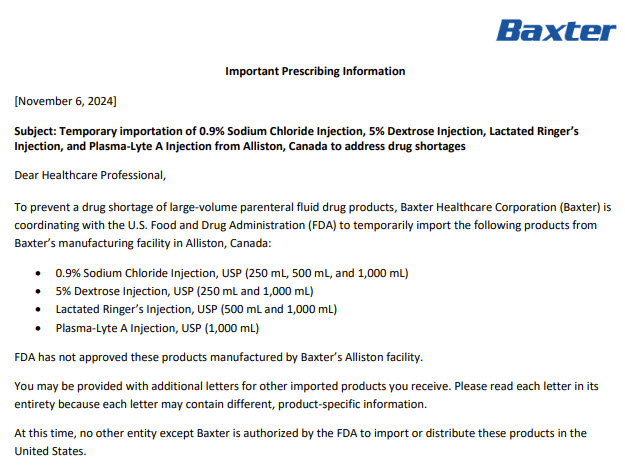
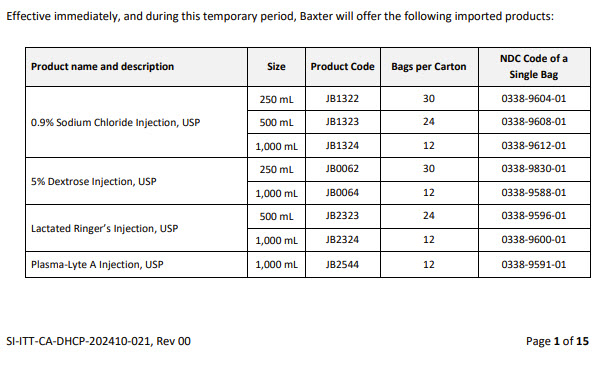
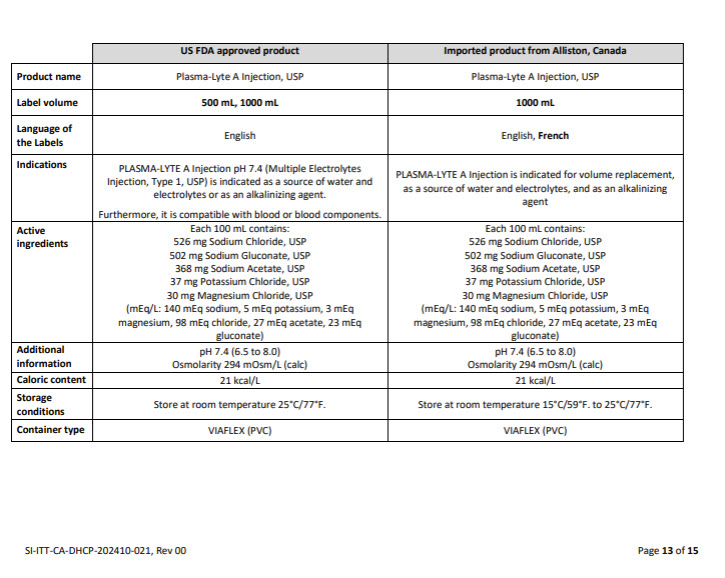
Label: LACTATED RINGERS- sodium chloride, potassium chloride, sodium lactate and calcium chloride injection, solution
- NDC Code(s): 0338-9596-01, 0338-9596-24, 0338-9600-01, 0338-9600-12
- Packager: Baxter Healthcare Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Health Care Professional Letter
Reportin Adverse Events or Product Quality Issues
To report adverse events associated with these imported products, please call Baxter at 1-866-888-2472, or fax: 1-800-759-1801. Adverse events or quality problems experienced with the use of these imported products may also be reported to the FDA’s MedWatch Adverse Event Reporting program either online, or by regulary mail or by fax:
- •
- Complete and submit the report Online: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program
- •
- Regular mail or Fax: Download form https://www.fda.gov/safety/medical-product-safety-information/medwatch-forms-fda-safety-reporting or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178)
To report product quality issues associated with these imported products, please contact Baxter Product Surveillance through Baxter – Product Feedback Portal (https://productfeedback.baxter.com/).
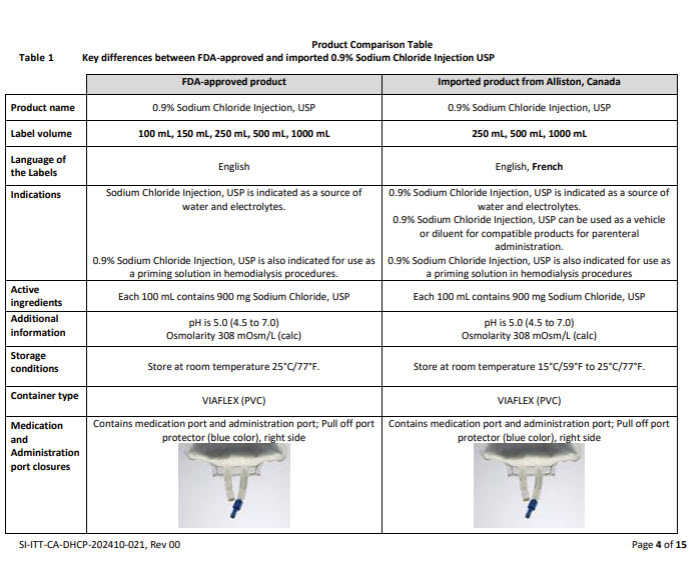
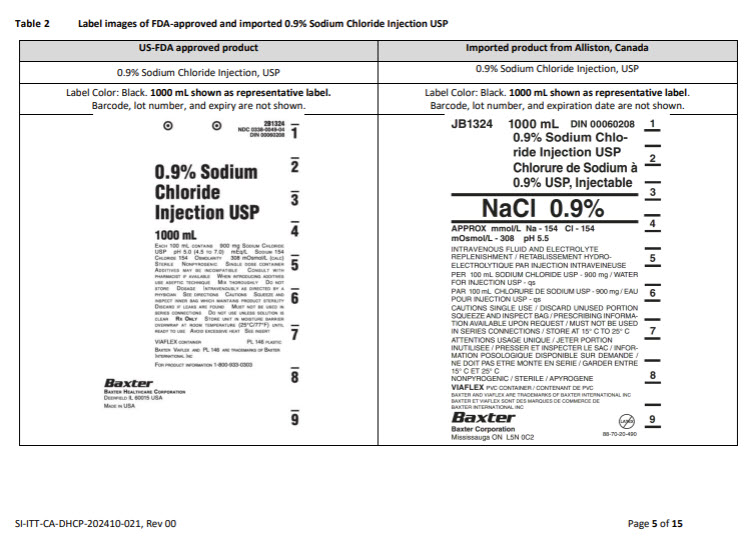
Please refer to the FDA-approved prescribing information for each drug product as follows:
- •
- 5% Dextrose Injection, USP (click DailyMed - DEXTROSE- dextrose monohydrate injection, solution)
- •
- 0.9% Sodium Chloride Injection, USP (click DailyMed - SODIUM CHLORIDE injection, solution)
- •
- Lactated Ringers Injection, USP (click DailyMed - LACTATED RINGERS- sodium chloride, potassium chloride, sodium lactate and calcium chloride injection, solution)
- •
- Plasma-Lyte Injection, USP (click DailyMed - PLASMA-LYTE A- sodium chloride, sodium gluconate, sodium acetate, potassium chloride and magnesium chloride injection, solution)
-
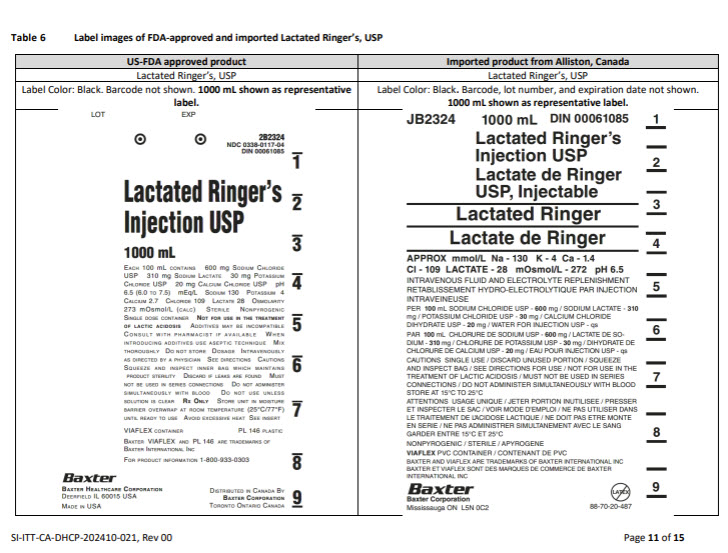
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
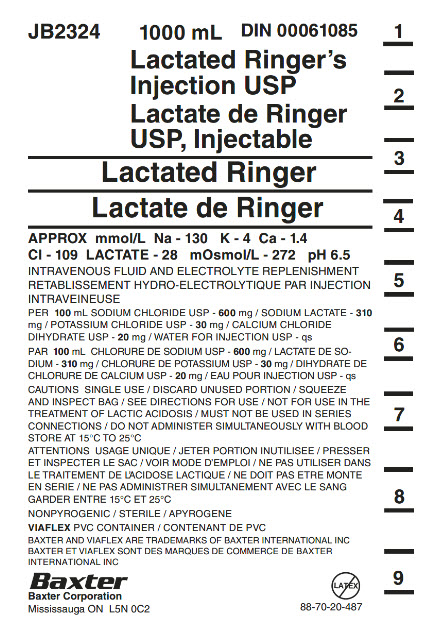
Container Label
JB2324
1000 mL
DIN 00061085Lactated Ringer’s
Injection USPLactate de Ringer
USP, InjectableLactated Ringer
Lactate de Ringer
APPROX mmol/L Na – 130 K – 4 Ca – 1.4
CI – 109 LACTATE – 28 mOsmol/L – 272 pH 6.5INTRAVENOUS FLUID AND ELECTROLYTE REPLENISHMENT
RETABLISSEMENT HYDRO-ELECTROLYTIQUE PAR INJECTION
INTRAVENIEUSEPER 100 mL SODIUM CHLORIDE USP – 600 mg / SODIUM LACTATE – 310
mg / POTASSIUM CHLORIDE USP – 30 mg / CALCIUM CHLORIDE
DIHYDRATE USP – 20 mg / WATER FOR INJECTION USP – qsPAR 100 mL CHLORURE DE SODIUM USP – 600 mg / LACTATE DE SO-
DIUM – 310 mg/ CHLORURE DE POTASSIUM USP – 30 mg / DIHYDRATE DE
CHLORURE DE CALCIUM USP – 20 mg / EAU POUR INJECTION USP – qsCAUTIONS SINGLE USE / DISCARD UNUSED PORTION / SQUEEZE
AND INSPECT BAG / SEE DIRECTIONS FOR USE / NOT FOR USE IN THE
TREATEMENT OF LACTIC ACIDOSIS / MUST NOT BE USED IN SERIES
CONNECTIONS / DO NOT ADMINSTER SIMULTANEOUSLY WITH BLOOD
STORE AT 15°C TO 25°CATTENTIONS USAGE UNIQUE / JETER PORTION INUTILISEE / PRESSER
ET INSPECTER LE SAC / VOIR MODE D’EMPLOI / NE PAS UTILISER DANS
LE TRAITEMENT DE L’ACIDOSE LACTIQUE / NE DOIT PAS ETRE MONTE
EN SERIE / NE PAS ADMINISTRER SIMULTANEMENT AVEC LE SANG
GARDER ENTRE 15°C ET 25°CNONPYROGENIC / STERILE / APYROGENE
VIAFLEX PVC CONTAINER / CONTENANT DE PVC
BAXTER AND VIAFLEX ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
BAXTER ET VIAFLEX SONT DES MARQUES DE COMMERCE DE BAXTER
INTERNATIONAL INCBaxter Logo
Baxter Corporation
Mississauga ON L5N 0C2No Latex Label
88-70-20-487
1
_
2
_
3
_
4
_
5
_
6
_
7
_
8
_
9
Container Label
JB2323
500 mL
DIN 00061085Lactated Ringer’s Injection
USPLactate de Ringer USP,
InjectableLactated Ringer
Lactate de Ringer
APPROX mmol/L Na – 130 K – 4 Ca – 1.4 CI
109 LACTATE – 28 mOsmol/L – 272 pH 6.5INTRAVENOUS FLUID AND ELECTROLYTE REPLENISHMENT / RETABLISSE-
MENT HYDRO-ELECTROLYTIQUE PAR INJECTION INTRAVENIEUSEPER100 mL SODIUM CHLORIDE USP – 600mg / SODIUM LACTATE – 310mb / PO-
TASSIUM CHLORIDE USP – 30mg / CALCIUM CHLORIDE DIHYDRATE USP – 20mg /
WATER FOR INJECTION USP – qsPAR100 mL CHLORURE DE SODIUM USP – 600mg / LACTATE DE SODIUM –
310mg/ CHLORURE DE POTASSIUM USP – 30mg / DIHYDRATE DECHLORURE DE
CALCIUM USP – 20mg / EAU POUR INJECTION USP – qsCAUTIONS SINGLE USE / DISCARD UNUSED PORTION / SQUEEZE AND INSPECT
BAG / SEE DIRECTIONS FOR USE / NOT FOR USE IN THE TREATEMENT OF LACTIC
ACIDOSIS / MUST NOT BE USED IN SERIES CONNECTIONS / DO NOT ADMINSTER
SIMULTANEOUSLY WITH BLOOD STORE AT 15°C TO 25°CATTENTIONS USAGE UNIQUE / JETER PORTION INUTILISEE / PRESSER ET INSPECT-
ER LE SAC / VOIR MODE D’EMPLOI / NE PAS UTILISER DANS LE TRAITEMENT DE
L’ACIDOSE LACTIQUE / NE DOIT PAS ETRE MONTE EN SERIE / NE PAS ADMINISTRER
SIMULTANEMENT AVEC LE SANG GARDER ENTRE 15°C ET 25°CNONPYROGENIC / STERILE / APYROGENE
VIAFLEX PVC CONTAINER / CONTENANT DE PVC
BAXTER AND VIAFLEX ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
BAXTER ET VIAFLEX SONT DES MARQUES DE COMMERCE DE BAXTER
INTERNATIONAL INCBaxter Logo
Baxter Corporation
Mississauga ON L5N 0C2No Latex Label
07-25-77-062
-1-
_
-2-
_
-3-
_
-4-
-
INGREDIENTS AND APPEARANCE
LACTATED RINGERS
sodium chloride, potassium chloride, sodium lactate and calcium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-9600 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 600 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 310 mg in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 30 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 20 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9600-12 12 in 1 CARTON 11/06/2024 1 NDC:0338-9600-01 1000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/06/2024 LACTATED RINGERS
sodium chloride, potassium chloride, sodium lactate and calcium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-9596 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 600 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 310 mg in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 30 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 20 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9596-24 24 in 1 CARTON 11/06/2024 1 NDC:0338-9596-01 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/06/2024 Labeler - Baxter Healthcare Company (005083209) Establishment Name Address ID/FEI Business Operations Baxter Corporation 205087968 ANALYSIS(0338-9600, 0338-9596) , LABEL(0338-9600, 0338-9596) , MANUFACTURE(0338-9600, 0338-9596) , STERILIZE(0338-9600, 0338-9596) , PACK(0338-9600, 0338-9596)