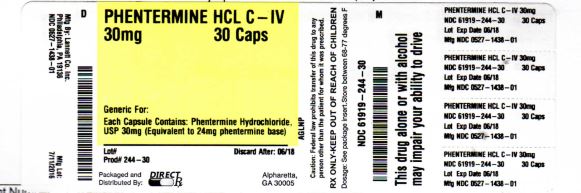
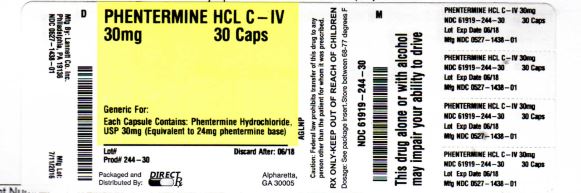
Label: PHENTERMINE HCL capsule
- NDC Code(s): 61919-244-30
- Packager: DIRECT RX
- This is a repackaged label.
- Source NDC Code(s): 0527-1438
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 11, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGE
Phentermine hydrochloride capsules are indicated as a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity for patients with an initial body mass index ≥ 30 kg/m2, or ≥ 27 kg/m2 in the presence of other risk factors (e.g., controlled hypertension, diabetes, hyperlipidemia).
Below is a chart of body mass index (BMI) based on various heights and weights.
BMI is calculated by taking the patient’s weight, in kilograms (kg), divided by the patient’s height, in meters (m), squared. Metric conversions are as follows: pounds ÷ 2.2 = kg; inches x 0.0254 = meters.
BODY MASS INDEX (BMI), kg/m2
Height (feet, inches)
Weight
(pounds) 5'0" 5'3" 5'6" 5'9" 6'0" 6'3"
140 27 25 23 21 19 18
150 29 27 24 22 20 19
160 31 28 26 24 22 20
170 33 30 28 25 23 21
180 35 32 29 27 25 23
190 37 34 31 28 26 24
200 39 36 32 30 27 25
210 41 37 34 31 29 26
220 43 39 36 33 30 28
230 45 41 37 34 31 29
240 47 43 39 36 33 30
250 49 44 40 37 34 31
The limited usefulness of agents of this class, including phentermine, [see Clinical Pharmacology (12.1, 12.2)] should be measured against possible risk factors inherent in their use such as those described below. -
DOSAGE & ADMINISTRATION
Exogenous Obesity
Dosage should be individualized to obtain an adequate response with the lowest effective dose.The usual adult dose is 15 mg to 30 mg as prescribed by the physician, at approximately 2 hours after breakfast for appetite control. Administration of one 30 mg capsule daily has been found to be adequate in depression of the appetite for 12 to 14 hours. Phentermine is not recommended for use in pediatric patients ≤ 16 years of age.
Late evening medication should be avoided because of the possibility of resulting insomnia.
-
DOSAGE FORMS & STRENGTHS
Phentermine hydrochloride capsules USP, 15 mg (equivalent to 12 mg phentermine base): powder-filled capsules, gray/orange; imprinted logo LANNETT on the cap and 1742 on the body.
Phentermine hydrochloride capsules USP, 30 mg (equivalent to 24 mg phentermine base): powder-filled capsules, natural/blue; imprinted logo LANNETT on the cap and 1308 on the body.
Phentermine hydrochloride capsules USP, 30 mg (equivalent to 24 mg phentermine base): powder-filled capsules, yellow/yellow; imprinted logo LANNETT on the cap and 1310 on the body.
Phentermine hydrochloride capsules USP, 30 mg (equivalent to 24 mg phentermine base): powder-filled capsules, black/black; imprinted logo LANNETT on the cap and logo 0597 logo on the body.
Phentermine hydrochloride capsules USP, 30 mg (equivalent to 24 mg phentermine base): pellet-filled capsules, natural/blue; imprinted logo LANNETT on the cap and 1438 on the body. -
CONTRAINDICATIONS
History of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure, uncontrolled hypertension)
During or within 14 days following the administration of monoamine oxidase inhibitors
Hyperthyroidism
Glaucoma
Agitated states
History of drug abuse
Pregnancy [see Use in Specific Populations (8.1)]
Nursing [see Use in Specific Populations (8.3)]
Known hypersensitivity, or idiosyncrasy to the sympathomimetic amines -
WARNINGS AND PRECAUTIONS
5.1 Coadministration with Other Drug Products for Weight Loss
Phentermine hydrochloride capsules are indicated only as short-term (a few weeks) monotherapy for the management of exogenous obesity. The safety and efficacy of combination therapy with phentermine and any other drug products for weight loss including prescribed drugs, over-the-counter preparations, and herbal products, or serotonergic agents such as selective serotonin reuptake inhibitors (e.g., fluoxetine, sertraline, fluvoxamine, paroxetine), have not been established. Therefore, coadministration of phentermine and these drug products is not recommended.5.2 Primary Pulmonary Hypertension
Primary Pulmonary Hypertension (PPH) - a rare, frequently fatal disease of the lungs - has been reported to occur in patients receiving a combination of phentermine with fenfluramine or dexfenfluramine. The possibility of an association between PPH and the use of phentermine alone cannot be ruled out; there have been rare cases of PPH in patients who reportedly have taken phentermine alone. The initial symptom of PPH is usually dyspnea. Other initial symptoms may include angina pectoris, syncope or lower extremity edema. Patients should be advised to report immediately any deterioration in exercise tolerance. Treatment should be discontinued in patients who develop new, unexplained symptoms of dyspnea, angina pectoris, syncope or lower extremity edema, and patients should be evaluated for the possible presence of pulmonary hypertension.5.3 Valvular Heart Disease
Serious regurgitant cardiac valvular disease, primarily affecting the mitral, aortic and/or tricuspid valves, has been reported in otherwise healthy persons who had taken a combination of phentermine with fenfluramine or dexfenfluramine for weight loss. The possible role of phentermine in the etiology of these valvulopathies has not been established and their course in individuals after the drugs are stopped is not known. The possibility of an association between valvular heart disease and the use of phentermine alone cannot be ruled out; there have been rare cases of valvular heart disease in patients who reportedly have taken phentermine alone.5.4 Development of Tolerance, Discontinuation in Case of Tolerance
When tolerance to the anorectant effect develops, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued.5.5 Effect on the Ability to Engage in Potentially Hazardous Tasks
Phentermine may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; the patient should therefore be cautioned accordingly.5.6 Risk of Abuse and Dependence
Phentermine is related chemically and pharmacologically to amphetamine (d- and dll-amphetamine) and other related stimulant drugs that have been extensively abused. The possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. See Drug Abuse and Dependence (9) and Overdosage (10).The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
5.7 Usage with Alcohol
Concomitant use of alcohol with phentermine may result in an adverse drug reaction.5.8 Use in Patients with Hypertension
Use caution in prescribing phentermine for patients with even mild hypertension (risk of increase in blood pressure).5.9 Use in Patients on Insulin or Oral Hypoglycemic Medications for Diabetes Mellitus
A reduction in insulin or oral hypoglycemic medications in patients with diabetes mellitus may be required. -
DRUG INTERACTIONS
7.1 Monoamine Oxidase Inhibitors
Use of phentermine is contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors because of the risk of hypertensive crisis.7.2 Alcohol
Concomitant use of alcohol with phentermine may result in an adverse drug reaction.7.3 Insulin and Oral Hypoglycemic Medications
Requirements may be altered [see Warnings and Precautions (5.9)].7.4 Adrenergic Neuron Blocking Drugs
Phentermine may decrease the hypotensive effect of adrenergic neuron blocking drugs. -
DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Phentermine is a Schedule IV controlled substance.9.2 Abuse
Phentermine is related chemically and pharmacologically to the amphetamines. Amphetamines and other stimulant drugs have been extensively abused and the possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program.9.3 Dependence
Abuse of amphetamines and related drugs may be associated with intense psychological dependence and severe social dysfunction. There are reports of patients who have increased the dosage of these drugs to many times than recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. A severe manifestation of chronic intoxication is psychosis, often clinically indistinguishable from schizophrenia. -
OVERDOSAGE
The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
10.1 Acute Overdosage
Manifestations of acute overdosage include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, and panic states. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmia, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Overdosage of pharmacologically similar compounds has resulted in fatal poisoning usually terminates in convulsions and coma.Management of acute phentermine hydrochloride intoxication is largely symptomatic and includes lavage and sedation with a barbiturate. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations in this regard. Acidification of the urine increases phentermine excretion. Intravenous phentolamine (Regitine®, CIBA) has been suggested on pharmacologic grounds for possible acute, severe hypertension, if this complicates overdosage.
10.2 Chronic Intoxication
Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. The most severe manifestation of chronic intoxications is psychosis, often clinically indistinguishable from schizophrenia. See Drug Abuse and Dependence (9.3). - DESCRIPTION
- NONCLINICAL TOXICOLOGY
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PHENTERMINE HCL
phentermine hcl capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61919-244(NDC:0527-1438) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENTERMINE HYDROCHLORIDE (UNII: 0K2I505OTV) (PHENTERMINE - UNII:C045TQL4WP) PHENTERMINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) D&C RED NO. 28 (UNII: 767IP0Y5NH) SHELLAC (UNII: 46N107B71O) ALCOHOL (UNII: 3K9958V90M) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) GELATIN (UNII: 2G86QN327L) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL ALCOHOL (UNII: Y4S76JWI15) FD&C RED NO. 40 (UNII: WZB9127XOA) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color blue ((blue/clear capsule filled with blue/white pellets)) , white ((blue/clear capsule filled with blue/white pellets)) Score score with uneven pieces Shape CAPSULE Size 18mm Flavor Imprint Code LANNETT;1438 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-244-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/11/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091359 07/11/2016 Labeler - DIRECT RX (079254320) Establishment Name Address ID/FEI Business Operations DIRECT RX 079254320 repack(61919-244)