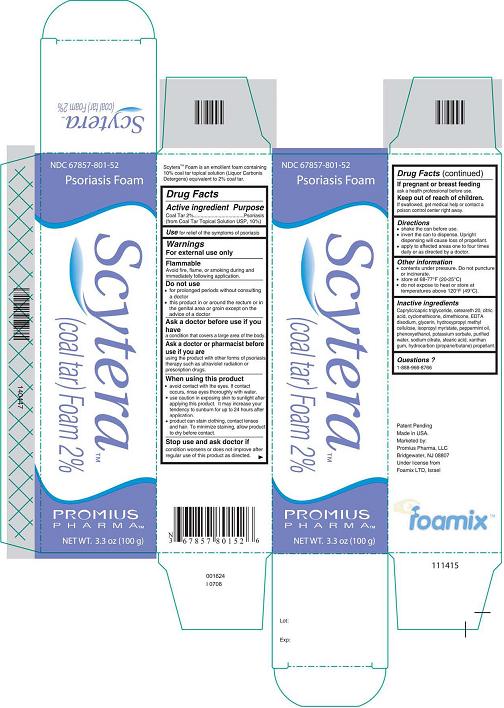
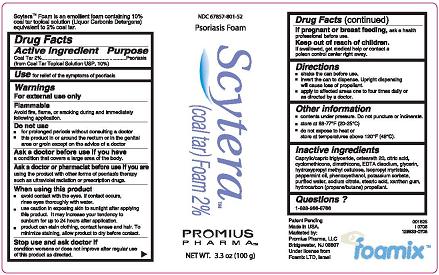
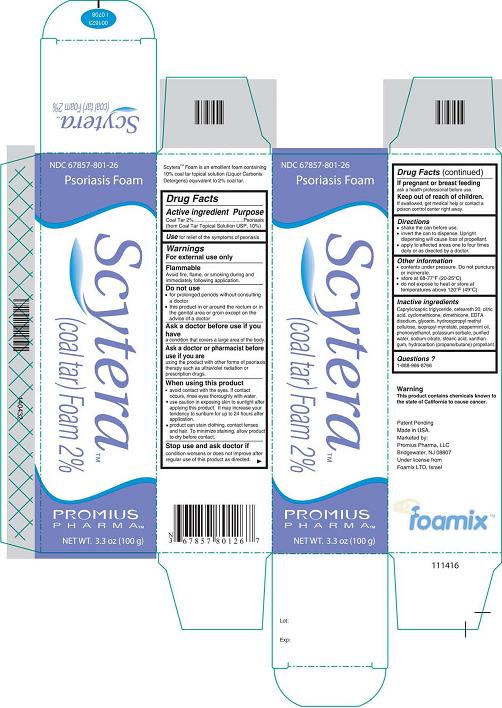
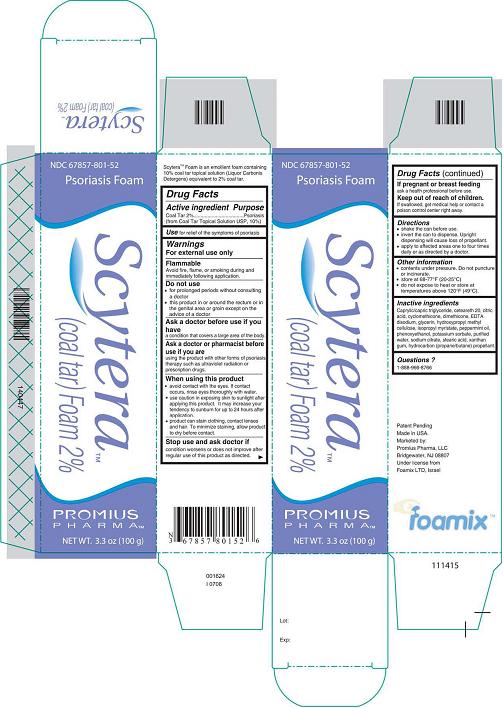
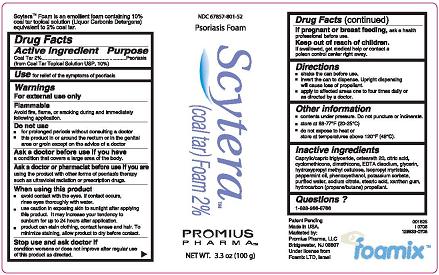
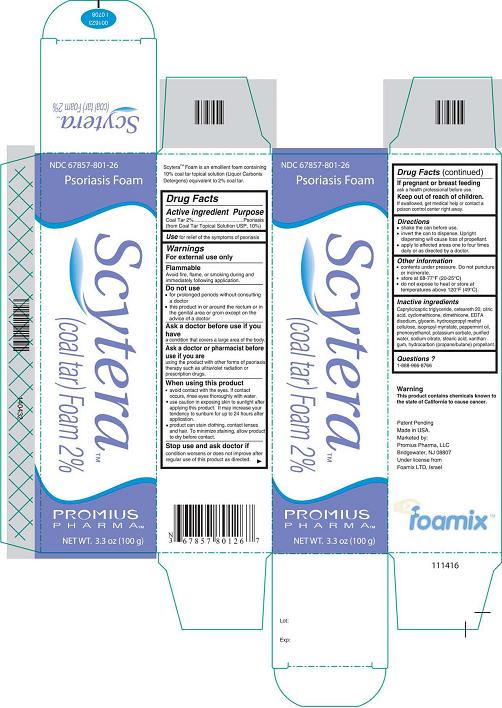
Label: SCYTERA (COAL TAR) FOAM, 2%- coal tar foam, 2% aerosol, foam
-
Contains inactivated NDC Code(s)
NDC Code(s): 63094-0801-1, 63094-0801-2, 63094-0801-3, 63094-0801-4 - Packager: DPT Laboratories, Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 18, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- Active Ingredient - Purpose
- Use
- Warnings
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
-
When using this product
-
avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with
water.
-
use caution in exposing skin to sunlight after applying this product. It
may increase your tendency to sunburn for up to 24 hours after application.
-
product can stain clothing, contact lenses, and hair. To minimize
staining, allow product to dry before contact.
-
avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with
water.
- Stop use and ask doctor if
- If pregnant or breastfeeding
- Keep out of reach of children.
- Directions
- Other information
-
Inactive Ingredients
Caprylic/capric triglyceride, ceteareth 20, citric acid, cyclomethicone, dimethicone, EDTA disodium, glycerin, hydroxypropyl methyl cellulose, isopropyl myristate, peppermint oil, phenoxyethanol, potassium sorbate, purified water, sodium citrate, stearic acid, xanthan gum, hydrocarbon (propane/butane) propellant.
- Questions?
- WARNINGS
- Scytera™ (Coal Tar)Foam 2%, 100 g
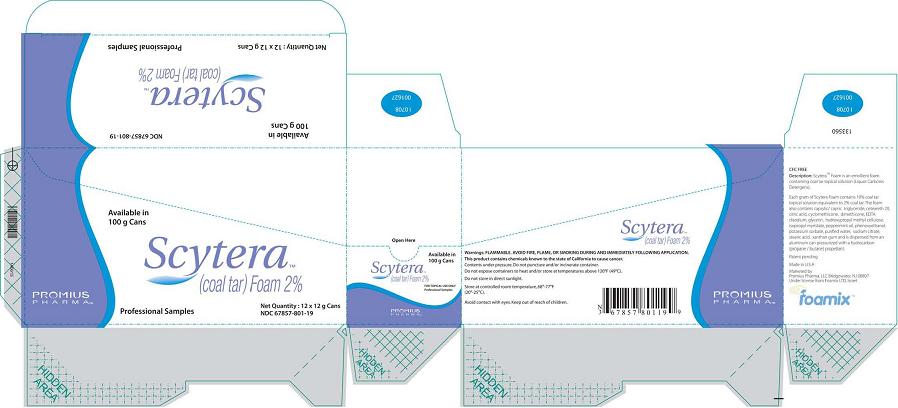
-
Scytera™ (Coal Tar)Foam 2%, 12 g Sample Size
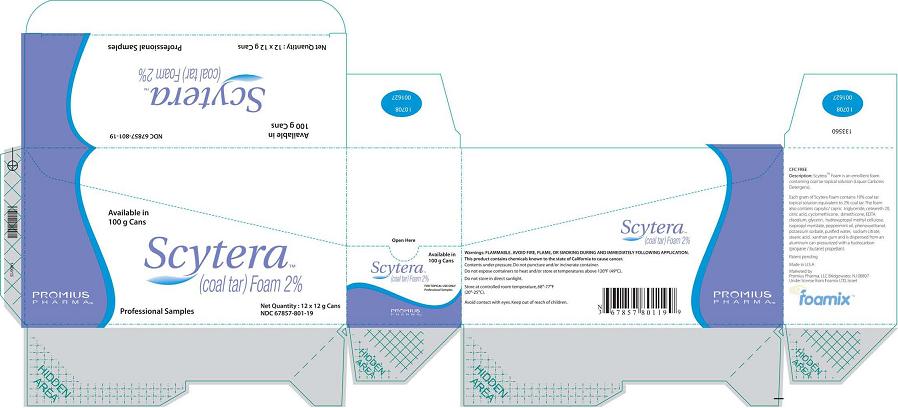
Packer (Box)
Available in 100 g Cans
Scytera™
(Coal Tar) Foam 2%
Promius Pharma™
Professional Samples
Net Quantity 12 x 12 g Cans
NDC 67857-801-12

Packer (Box) (California)
Available in 100 g Cans
Scytera™
(Coal Tar) Foam 2%
Promius Pharma™
Professional Samples
Net Quantity 12 x 12 g Cans
NDC 67857-801-19
Can Label
NDC 67857-801-12
Psoriasis Foam
Scytera™
(Coal Tar) Foam 2%
SAMPLE, NOT FOR SALE
NET WT. 0.4 oz (12 g)

-
INGREDIENTS AND APPEARANCE
SCYTERA (COAL TAR) FOAM, 2%
coal tar foam, 2% aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63094-0801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAL TAR (UNII: R533ESO2EC) (COAL TAR - UNII:R533ESO2EC) COAL TAR 20 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSE (UNII: 3NXW29V3WO) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) STEARIC ACID (UNII: 4ELV7Z65AP) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEPPERMINT OIL (UNII: AV092KU4JH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63094-0801-1 1 in 1 CARTON 1 100 g in 1 CAN 2 NDC:63094-0801-2 12 in 1 BOX 2 12 g in 1 CAN 3 NDC:63094-0801-3 1 in 1 CARTON 3 NDC:63094-0801-1 100 g in 1 CAN 4 NDC:63094-0801-4 12 in 1 BOX 4 NDC:63094-0801-2 12 g in 1 CAN Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358 10/31/2008 Labeler - DPT Laboratories, Ltd. (621782218)