Scytera™ Foam is an emollient foam containing 10% coal tar topical
solution (Liquor Carbonis Detergens) equivalent to 2% coal tar.
Active Ingredient - Purpose
Active ingredient: Coal Tar 2% (from Coal Tar Topical Solution USP, 10%)Purpose: Psoriasis
Warnings
For external use only.Flammable
Avoid fire, flame, or smoking during and immediately following application.
Do not use
- for prolonged periods without consulting a doctor
- this product in or around the rectum or in the genital area or groin except on the advice of a doctor
Ask a doctor or pharmacist before use if you are
using the product with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs.When using this product
-
avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with
water.
-
use caution in exposing skin to sunlight after applying this product. It
may increase your tendency to sunburn for up to 24 hours after application.
-
product can stain clothing, contact lenses, and hair. To minimize
staining, allow product to dry before contact.
Stop use and ask doctor if
condition worsens or does not improve after regular use of this product as directed.Keep out of reach of children.
If swallowed, get medical help or contact a poison control center right away.Directions
-
shake the can well before use.
-
invert the can to dispense. Upright dispensing will cause loss of
propellant.
-
apply to affected areas one to four times daily or as directed by a doctor.
Other information
- contents under pressure. Do not puncture or incinerate.
- store at 68°F to 77°F (20°C to 25°C)
- do not expose to heat or store at temperatures above 120°F (49°C)
Inactive Ingredients
Caprylic/capric triglyceride, ceteareth 20, citric acid, cyclomethicone, dimethicone, EDTA disodium, glycerin, hydroxypropyl methyl cellulose, isopropyl myristate, peppermint oil, phenoxyethanol, potassium sorbate, purified water, sodium citrate, stearic acid, xanthan gum, hydrocarbon (propane/butane) propellant.
Warning
This product contains chemicals known to the state of California to cause cancer.
Patent Pending
Made in USA.
Marketed by:
Promius Pharma, LLC
Bridgewater, NJ 08807
Under license from
Foamix LTD, Israel
This product contains chemicals known to the state of California to cause cancer.
Patent Pending
Made in USA.
Marketed by:
Promius Pharma, LLC
Bridgewater, NJ 08807
Under license from
Foamix LTD, Israel
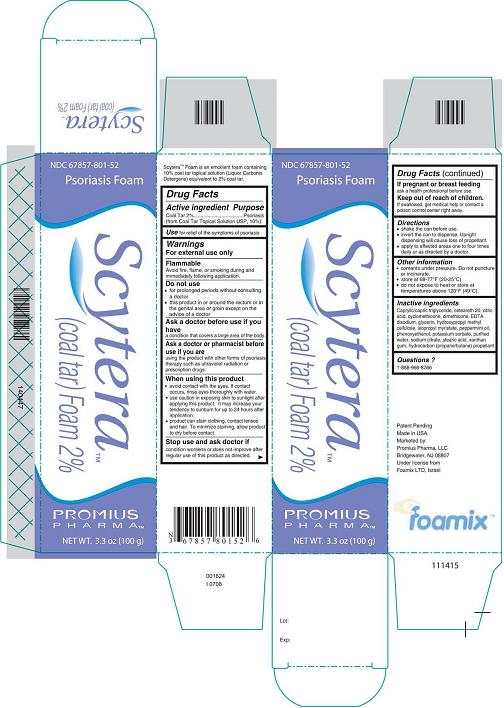
Scytera™ (Coal Tar)Foam 2%, 100 g
Carton and Can LabelNDC 67857-801-52
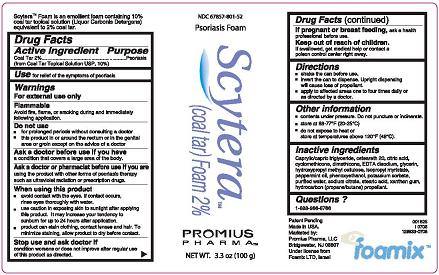
Psoriasis Foam
Scytera™
(Coal Tar) Foam 2%
Promius Pharma™
NET WT. 3.3 oz (100 g)
Carton (California)
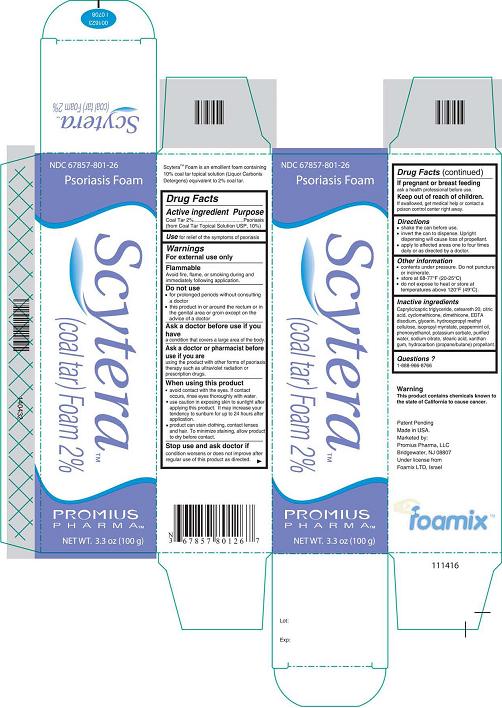
NDC 67857-801-26
Psoriasis Foam
Scytera™
(Coal Tar) Foam 2%
Promius Pharma™
NET WT. 3.3 oz (100 g)
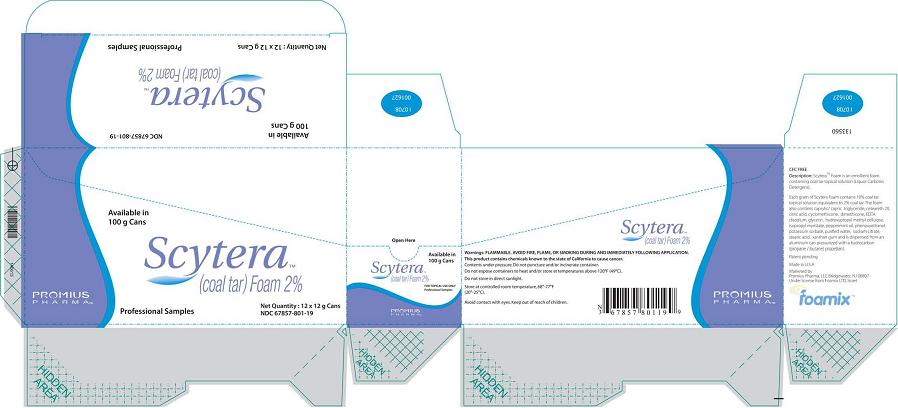
Scytera™ (Coal Tar)Foam 2%, 12 g Sample Size
Packer (Box)Available in 100 g Cans
Scytera™
(Coal Tar) Foam 2%
Promius Pharma™
Professional Samples
Net Quantity 12 x 12 g Cans
NDC 67857-801-12

Packer (Box) (California)
Available in 100 g Cans
Scytera™
(Coal Tar) Foam 2%
Promius Pharma™
Professional Samples
Net Quantity 12 x 12 g Cans
NDC 67857-801-19
Can Label
NDC 67857-801-12
Psoriasis Foam
Scytera™
(Coal Tar) Foam 2%
SAMPLE, NOT FOR SALE
NET WT. 0.4 oz (12 g)
