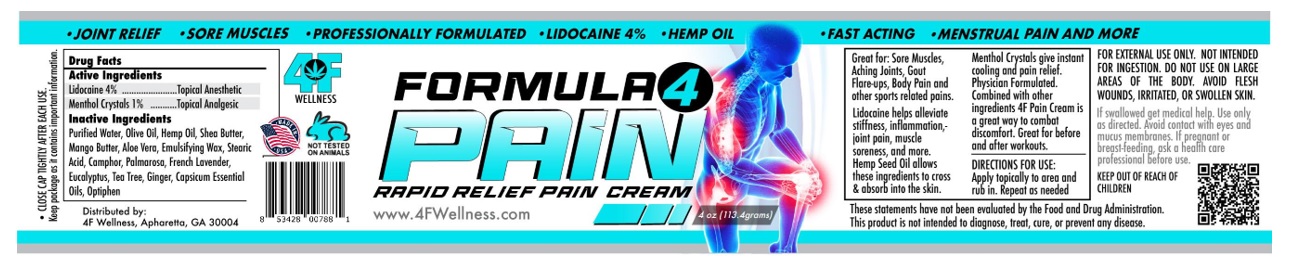
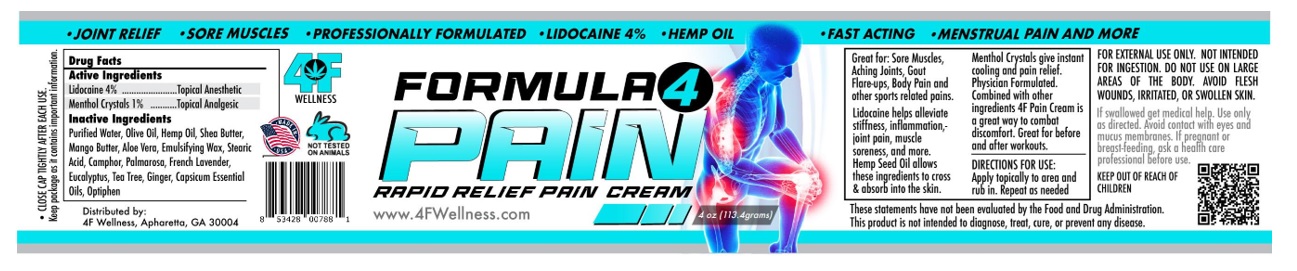
Label: FORMULA 4 PAIN- lidocaine hcl, menthol cream
- NDC Code(s): 58292-005-01
- Packager: Lifequest Creations LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Direction
- Inactive ingredients
-
Other information
Great for : Sore muscles, aching joints, gout flare-ups and body pain and other sports related pain.
Menthol crystals gives instant cooling and pain releif. Physician formulated (roll-on-blend) glides on is non-sticky, and absorbs quickly.
Arnica reduces bruising and swelling. Lidocaine helps alleviate stiffness, inflammation, joint pain, muscle soreness and more. Hemp seed oil
allows ingredients to cross and absorb into the skin. With its healing properties, Emu oil reduces fibrosis from skin damage caused by radiation
therapy.
- Product label
-
INGREDIENTS AND APPEARANCE
FORMULA 4 PAIN
lidocaine hcl, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58292-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) OLIVE OIL (UNII: 6UYK2W1W1E) SHEA BUTTER (UNII: K49155WL9Y) MANGO (UNII: I629I3NR86) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PALMAROSA OIL (UNII: 0J3G3O53ST) TEA TREE OIL (UNII: VIF565UC2G) EUCALYPTUS OIL (UNII: 2R04ONI662) CAMPHOR (NATURAL) (UNII: N20HL7Q941) LAVENDER OIL (UNII: ZBP1YXW0H8) GINGER OIL (UNII: SAS9Z1SVUK) CAPSICUM OLEORESIN (UNII: UW86K581WY) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) ARNICA MONTANA WHOLE (UNII: O80TY208ZW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58292-005-01 113 g in 1 JAR; Type 0: Not a Combination Product 05/07/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/07/2023 Labeler - Lifequest Creations LLC (053654857)