Label: ORLADEYO- berotralstat hydrochloride capsule
ORLADEYO- berotralstat hydrochloride pellet
-
NDC Code(s):
72769-101-01,
72769-102-01,
72769-111-02,
72769-112-02, view more72769-113-02, 72769-114-02
- Packager: BioCryst Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ORLADEYO® safely and effectively. See full prescribing information for ORLADEYO.
ORLADEYO (berotralstat) capsules, for oral use
ORLADEYO (berotralstat) oral pellets
Initial U.S. Approval: 2020RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Recommended dosage in adult and pediatric patients aged 12 years and older: 150 mg capsule orally once daily with food. (2.1)
- Recommended dosage in pediatric patients aged 2 to less than 12 years is based on body weight as follows (2.2, 2.5):
Weight Recommended Dosage
(oral pellets)Administration Instructions 12 kg to less than 24 kg 72 mg once daily Pour directly in mouth and swallow immediately with non-acidic liquid, or,
Sprinkle over 1 tablespoon (15 mL) of non-acidic soft food and consume immediately.
A meal should be consumed just before or after administration.24 kg to less than 32 kg 96 mg once daily 32 kg to less than 40 kg 108 mg once daily 40 kg or greater 132 mg once daily DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
An increase in QTc interval prolongation can occur at dosages higher than the recommended dosage. Additional doses or doses of ORLADEYO higher than the recommended dosage are not recommended. (5.1)
ADVERSE REACTIONS
Most common adverse reactions (≥10%) are abdominal pain, vomiting, diarrhea, back pain, and gastroesophageal reflux disease. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact BioCryst Pharmaceuticals, Inc. at 1-833-633-2279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults and Pediatric Patients 12 Years of Age and Older
2.2 Recommended Dosage in Pediatric Patients 2 Years to Less than 12 Years of Age
2.3 Recommended Dosage in Patients with Hepatic Impairment
2.4 Dosage Modification in Patients with Persistent GI Adverse Reactions
2.5 Administration Instructions for Oral Pellets
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of QTc Interval Prolongation with Higher-Than-Recommended Dosages
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on ORLADEYO
7.2 Effect of ORLADEYO on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ORLADEYO® is indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and pediatric patients 2 years of age and older.
Limitations of Use:
The safety and effectiveness of ORLADEYO for the treatment of acute HAE attacks have not been established. ORLADEYO should not be used for treatment of acute HAE attacks. Additional doses or doses of ORLADEYO higher than the prescribed once-daily dose are not recommended due to the potential for QTc interval prolongation [see Warnings and Precautions (5.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults and Pediatric Patients 12 Years of Age and Older
The recommended dosage of ORLADEYO capsules for adults and pediatric patients 12 years of age and older is 150 mg taken orally once daily with food.
2.2 Recommended Dosage in Pediatric Patients 2 Years to Less than 12 Years of Age
The recommended dosage of ORLADEYO oral pellets for pediatric patients 2 years to less than 12 years of age is based on the patient's body weight as provided in Table 1. Take ORLADEYO orally with food (a meal should be consumed just before or after dosing) [see Dosage and Administration (2.5)].
Table 1: Recommended Dosage of ORLADEYO Oral Pellets by Body Weight in Pediatric Patients 2 Years to Less than 12 Years of Age Body Weight (kg) Recommended Dosage of ORLADEYO Oral Pellets 12 kg to less than 24 kg 72 mg (one packet) once daily 24 kg to less than 32 kg 96 mg (one packet) once daily 32 kg to less than 40 kg 108 mg (one packet) once daily 40 kg or greater 132 mg (one packet) once daily 2.3 Recommended Dosage in Patients with Hepatic Impairment
Mild Hepatic Impairment (Child-Pugh Class A)
Adult and Pediatric Patients 2 Years of Age and Older
- No dosage modification of ORLADEYO is recommended [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Moderate or Severe Hepatic Impairment (Child-Pugh Class B or C)
Adult and Pediatric Patients 12 Years of Age and Older
- Recommended dosage of ORLADEYO capsules is 110 mg taken orally once daily with food [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Pediatric Patients 2 Years to Less than 12 Years of Age
- Avoid use of ORLADEYO [see Use in Specific Populations (8.7)].
2.4 Dosage Modification in Patients with Persistent GI Adverse Reactions
Gastrointestinal (GI) reactions may occur in patients receiving ORLADEYO [see Adverse Reactions (6.1)].
- For adults and pediatric patients 12 years of age and older, if GI adverse reactions persist, consider a lower ORLADEYO capsules dosage of 110 mg once daily with food.
- For pediatric patients 2 to less than 12 years of age with persistent GI adverse reactions, consider the risks and benefits of continuing treatment with ORLADEYO.
2.5 Administration Instructions for Oral Pellets
Do not chew or crush ORLADEYO oral pellets because this will affect the film coating (taste masking) and result in bitter taste. Administer one packet of oral pellets as follows:
- Pour the entire contents of one packet directly into the mouth and swallow immediately with non-acidic liquid (e.g., water or milk).
OR
- Sprinkle the entire contents of one packet over approximately one tablespoon (15 mL) of soft, non-acidic food and consume immediately. Food should be at or below room temperature. Examples of soft, non-acidic foods include pudding, mashed potatoes, creamed corn, pureed peas, pureed bananas, and pureed carrots. Acidic foods such as yogurt and applesauce should not be used because they can dissolve the film coating (taste masking) and result in a bitter taste. The film coating (taste masking) remains intact for 10 minutes. If the pellets are not consumed within 10 minutes of sprinkling over food, they should be discarded.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of QTc Interval Prolongation with Higher-Than-Recommended Dosages
ORLADEYO should not be used for treatment of acute attacks of HAE. Additional doses or doses of ORLADEYO higher than the recommended dosage are not recommended. An increase in QTc interval was observed in adults at dosages higher than 150 mg once daily and was concentration dependent [see Dosage and Administration (2) and Clinical Pharmacology (12.2)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
- QTc Interval Prolongation [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adult and Pediatric Patients 12 Years of Age and Older
The safety of ORLADEYO is primarily based on 24-week (Part 1) data from a 3-part, double-blind, parallel-group, placebo-controlled trial (Trial 1) in 120 patients with Type I or II HAE who were randomized and dosed with either ORLADEYO 110 mg, 150 mg, or placebo, once daily with food. After Week 24, patients who continued in the study received active treatment through 48 weeks.
In Trial 1, a total of 81 patients aged 12 years and older with HAE received at least one dose of ORLADEYO in Part 1. Overall, 66% of patients were female and 93% of patients were White with a mean age of 41.6 years. The proportion of patients who discontinued study drug prematurely due to adverse reactions was 7% and 3% for patients treated with ORLADEYO 110 mg and 150 mg, respectively, and 3% for placebo-treated patients. No deaths occurred in the trial.
The safety profile of ORLADEYO was generally similar across all subgroups of patients, including analysis by age, sex, and geographic region.
Table 2 shows adverse reactions occurring in ≥10% of adult and pediatric patients aged 12 years and older in any ORLADEYO treatment group that also occurred at a higher rate than in the placebo treatment group in Trial 1.
Table 2: Adverse Reactions Observed in ≥10% of Adult and Pediatric Patients Aged 12 Years and Older with HAE in Any ORLADEYO Treatment Group (Trial 1) Adverse Reaction Placebo
(N=39)ORLADEYO 110 mg
(N=41)150 mg
(N=40)Total
(N=81)n (%) n (%) n (%) n (%) Abdominal Pain* 4 (10) 4 (10) 9 (23) 13 (16) Vomiting 1 (3) 4 (10) 6 (15) 10 (12) Diarrhea† 0 4 (10) 6 (15) 10 (12) Back Pain 1 (3) 1 (2) 4 (10) 5 (6) Gastroesophageal Reflux Disease 0 4 (10) 2 (5) 6 (7) Gastrointestinal adverse reactions, including abdominal pain, vomiting, and diarrhea occurred more frequently in patients receiving ORLADEYO 150 mg versus ORLADEYO 110 mg or placebo. These adverse reactions generally occurred early after initiation of treatment with ORLADEYO, became less frequent with time, and typically self-resolved. No patients in the ORLADEYO 150 mg dose group and 1 patient in the ORLADEYO 110 mg dose group discontinued treatment due to a gastrointestinal adverse reaction.
Less Common Adverse Reactions
Other adverse reactions that occurred in Part 1 of Trial 1 with an incidence between 5% to <10% and at a higher incidence in ORLADEYO-treated patients compared to placebo-treated patients included headache (9% versus 5%), fatigue (6% versus 3%), and flatulence (6% versus 3%).
A maculopapular drug rash was reported in less than 1% of patients treated with ORLADEYO. The rash resolved, including in patients who continued dosing.
Safety data are also available from 227 patients enrolled in an ongoing, open-label, long-term safety study (Trial 2) who received ORLADEYO 110 mg (N=100) or 150 mg (N=127) once daily with food and are consistent with the 24-week controlled safety data from Trial 1 (Part 1).
Laboratory Abnormalities
Transaminase Elevations
In Part 1 of Trial 1, one patient treated with ORLADEYO 150 mg discontinued treatment due to asymptomatic elevated transaminases (ALT >8x the upper limit of normal [ULN] and AST >3x ULN). Total bilirubin was normal. No patient receiving ORLADEYO 110 mg or placebo developed transaminase levels >3x ULN. In addition to this patient, 2 ORLADEYO-treated patients developed laboratory-related hepatic adverse reactions compared to 1 placebo-treated patient. No patient reported serious adverse reactions of elevated transaminases.
Adverse Reactions in Pediatric Patients 2 to Less than 12 Years of Age
The safety of ORLADEYO was evaluated in 29 pediatric patients aged 2 to <12 years with HAE in a multicenter, single-arm, open-label safety and pharmacokinetic study (Trial 3). Patients received ORLADEYO based on patient's body weight for at least 12 weeks, with 17 patients completing 48 weeks of treatment. No new safety signals were observed in these patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ORLADEYO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: nausea
-
7 DRUG INTERACTIONS
7.2 Effect of ORLADEYO on Other Drugs
CYP2D6 and CYP3A4 Substrates
ORLADEYO at a dose of 150 mg is a moderate inhibitor of CYP2D6 and CYP3A4. Concomitant use of ORLADEYO with CYP2D6 or CYP3A4 substrates can increase exposure of the CYP2D6 or CYP3A4 substrates and may increase the risk of adverse reactions associated with the substrates. If ORLADEYO is concomitantly used with CYP2D6 or CYP3A4 substrates where minimal increases in the concentration of the substrates may lead to serious adverse reactions, closely monitor or modify the dosage of the CYP2D6 or CYP3A4 substrate [see Clinical Pharmacology (12.3)]. Refer to the Prescribing Information of the CYP2D6 or CYP3A4 substrate.
Desogestrel
Concomitant use of ORLADEYO with desogestrel increases exposure to etonogestrel, the active metabolite of desogestrel, and may increase the risk of desogestrel-associated adverse reactions. Consider the benefits and risks when using ORLADEYO concomitantly with desogestrel [see Clinical Pharmacology (12.3)].
P-gp Substrates
ORLADEYO at 2-times the maximum recommended dose of 150 mg is a P-gp inhibitor and can increase exposure of P-gp substrates, leading to increased risk of adverse reactions associated with the substrates. Higher-than-recommended dosages of ORLADEYO are not recommended [see Warnings and Precautions (5.1)]. If ORLADEYO is concomitantly used with P-gp substrates where minimal increases in the concentration of the substrates may lead to serious adverse reactions, closely monitor or modify the dosage of the P-gp substrate [see Clinical Pharmacology (12.3)]. Refer to the Prescribing Information of the P-gp substrate.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient data in pregnant women available to inform drug-related risks with ORLADEYO use in pregnancy. Based on animal reproduction studies, no evidence of structural alterations was observed when berotralstat was administered orally to pregnant rats and rabbits during organogenesis at doses up to approximately 10- and 2-times, respectively, the maximum recommended human daily dose (MRHDD) in adults on an AUC basis (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In animal reproduction studies, oral administration of berotralstat to pregnant rats and rabbits during the period of organogenesis did not cause fetal structural alterations. The berotralstat dose in rats and rabbits was up to approximately 10- and 2-times, respectively, the MRHDD in adults (on an AUC basis at maternal doses of 75 and 100 mg/kg/day, respectively). In a pre- and postnatal development study in rats, oral administration of berotralstat to pregnant rats during the period of organogenesis and until delivery at doses up to 45 mg/kg/day (approximately 2-times of the MRHDD on a mg/m2 basis) did not cause fetal structural alterations either. Berotralstat concentrations in the fetal blood were approximately 5% to 11% of the maternal blood.
8.2 Lactation
Risk Summary
There are no data on the presence of berotralstat in human milk, its effects on the breastfed infant, or its effects on milk production. However, when a drug is present in animal milk, it is likely that the drug will be present in human milk. Low levels of berotralstat were detected in the plasma of rat pups when dams were dosed with the drug orally during the lactation period. The berotralstat concentration in the pup plasma was approximately 2% of the maternal plasma (see Data).
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ORLADEYO and any potential adverse effects on the breastfed infant from ORLADEYO or from the underlying maternal condition.
Data
Animal Data
In the pre- and post-natal development study in rats, berotralstat was administered to dams during the pregnancy and lactation periods at doses up to 45 mg/kg/day (approximately 2-times of the MRHDD on a mg/m2 basis). Berotralstat was detected in the plasma of pups during the lactation period. The berotralstat concentration in the pup plasma was approximately 2% of the maternal plasma. Both dams and pups at 45 mg/kg/day showed statistically significant decreases in body weight gain (p<0.05). No treatment-related effects were observed at 25 mg/kg/day (approximately equal to the MRHDD on a mg/m2 basis).
8.4 Pediatric Use
The safety and effectiveness of ORLADEYO for prophylaxis to prevent attacks of hereditary angioedema have been established in pediatric patients aged 2 and older. The use of ORLADEYO in pediatric patients aged 12 to <18 years with HAE is supported by evidence from an adequate and well-controlled trial (Trial 1) that included adults and a total of 6 pediatric patients aged 12 to <18 years. The safety profile and attack rate in the study were similar to those observed in adults [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)]. An additional 10 pediatric patients aged 12 to <18 years were enrolled in the open-label study (Trial 2).
The use of ORLADEYO in pediatric patients aged 2 to <12 years with HAE is supported by efficacy data from an adequate and well-controlled study in adults and pediatric patients aged 12 years and older (Trial 1), and pharmacokinetic and safety data from 29 pediatric patients aged 2 to less than 12 years enrolled in a multicenter, single-arm, open-label study with additional support from population pharmacokinetic analyses. Pediatric patients aged 2 to less than 12 years received a dosage of ORLADEYO based on the patient's body weight, which showed no clinically significant difference in drug exposures from those observed in adults treated with ORLADEYO 150 mg [see Clinical Pharmacology (12.3)]. Pediatric patients aged 2 to less than 12 years were treated for at least 12 weeks, with 17 patients completing 48 weeks of treatment. No new safety signals were observed in pediatric patients aged 2 to less than 12 years [see Adverse Reactions (6.1)].
The safety and effectiveness of ORLADEYO have not been established in pediatric patients younger than 2 years of age.
8.5 Geriatric Use
Of the total number of patients in clinical studies of ORLADEYO, 9 patients were 65 years of age and over in Trial 1, and 5 patients were 65 years of age and over in Trial 2 (open-label safety study). No overall differences in safety or effectiveness of ORLADEYO have been observed between patients 65 years of age and older and younger adult patients [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
8.6 Renal Impairment
Mild to Moderate Renal Impairment:
Adult and Pediatric Patients Aged 2 Years and Older
- No dosage modification of ORLADEYO is recommended [see Clinical Pharmacology (12.3)].
Severe Renal Impairment:
End-Stage Renal Disease:
ORLADEYO has not been studied in patients with End-Stage Renal Disease (CLCR <15 mL/min or eGFR <15 mL/min/1.73 m2 or patients requiring hemodialysis) and therefore is not recommended for use in these patient populations [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Mild Hepatic Impairment (Child-Pugh Class A):
Adult and Pediatric Patients Aged 2 Years and Older
- No dosage modification of ORLADEYO is recommended [see Clinical Pharmacology (12.3)].
Moderate or Severe Hepatic Impairment (Child-Pugh B or C):
Adult and Pediatric Patients Aged 12 Years and Older
- The recommended dosage of ORLADEYO capsules is 110 mg once daily with food [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
ORLADEYO (berotralstat) is a plasma kallikrein inhibitor. Berotralstat is presented as the dihydrochloride salt with the chemical name 1-[3-(aminomethyl)phenyl]-N-(5-{(R)-(3-cyanophenyl)[(cyclopropylmethyl)amino]methyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide dihydrochloride. The chemical structure is:
Berotralstat dihydrochloride is a white to off-white powder that is soluble in water at pH ≤4. The molecular formula is C30H26F4N6O ∙ 2HCl and the molecular weight is 635.49 (dihydrochloride).
ORLADEYO (berotralstat) capsules contain 150 mg of berotralstat (equivalent to 169.4 mg berotralstat dihydrochloride) or 110 mg of berotralstat (equivalent to 124.3 mg berotralstat dihydrochloride) in hard gelatin capsules for oral administration. Each capsule contains the active ingredient berotralstat dihydrochloride and the inactive ingredients colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starch.
ORLADEYO (berotralstat) oral pellets are white to off-white film-coated pellets for oral administration and enclosed in a unit-dose packet containing berotralstat 72 mg (equivalent to 81.3 mg berotralstat dihydrochloride), 96 mg (equivalent to 108.4 mg berotralstat dihydrochloride), 108 mg (equivalent to 122.0 mg berotralstat dihydrochloride), or 132 mg (equivalent to 149.1 mg of berotralstat dihydrochloride). Each unit-dose packet contains the active ingredient berotralstat dihydrochloride and the inactive ingredients colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starch. The oral pellets film coating contains butylated methacrylate copolymer, silicon dioxide, sodium lauryl sulphate, stearic acid, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Berotralstat is a plasma kallikrein inhibitor that binds to plasma kallikrein and inhibits its proteolytic activity. Plasma kallikrein is a protease that cleaves high-molecular-weight kininogen (HMWK) to generate cleaved HMWK (cHMWK) and bradykinin, a potent vasodilator that increases vascular permeability resulting in swelling and pain associated with HAE. In patients with HAE due to C1-inhibitor (C1-INH) deficiency or dysfunction, normal regulation of plasma kallikrein activity is not present, which leads to uncontrolled increases in plasma kallikrein activity and results in angioedema attacks. Berotralstat decreases plasma kallikrein activity to control excess bradykinin generation in patients with HAE.
12.2 Pharmacodynamics
Concentration-dependent inhibition of plasma kallikrein, measured as a reduction from baseline of specific enzyme activity, was demonstrated after oral administration of ORLADEYO once daily in patients with HAE.
Cardiac Electrophysiology
At the maximum recommended dosage of 150 mg once daily in adults, clinically significant QTc interval prolongation was not observed . At 3-times the maximum recommended dosage, the mean (upper 90% confidence interval) increase in QTcF was 15.9 msec (23.5 msec). The observed increase in QTcF was concentration dependent.
12.3 Pharmacokinetics
Following oral administration of berotralstat 150 mg once daily in adults, the geometric mean steady-state Cmax is 158 ng/mL (range: 110 to 234 ng/mL) and steady-state area under the curve over the dosing interval (AUCtau) is 2770 ng*hr/mL (range: 1880 to 3790 ng*hr/mL). Following oral administration of berotralstat 110 mg once daily, the geometric mean steady-state Cmax is 97.8 ng/mL (range: 63 to 235 ng/mL) and steady-state AUCtau is 1600 ng*hr/mL (range: 950 to 4170 ng*hr/mL).
Berotralstat exposure (Cmax and AUC) increases in a greater than dose-proportional manner and steady state is reached in approximately 6 to 12 days. Berotralstat accumulation at steady state is approximately 5-fold at the recommended dosage.
No clinically significant difference in the pharmacokinetics of berotralstat was observed between healthy adult subjects and patients with HAE.
Absorption
The median time to maximum plasma concentration (Tmax) of berotralstat when administered with food is 5 hours (range: 1 to 8 hours).
No clinically significant difference in ORLADEYO exposure was observed following oral administration of either the pellets or capsules at the recommended adult dosage for 7 days.
Distribution
Plasma protein binding is approximately 99%. After a single dose of radiolabeled berotralstat at 2-times the maximum recommended dosage of 150 mg, the blood to plasma ratio was approximately 0.92.
Elimination
The median elimination half-life of berotralstat is approximately 93 hours (range: 39 to 152 hours).
Metabolism
Berotralstat is metabolized by CYP2D6 and by CYP3A4 with low turnover in vitro. After a single oral dose of radiolabeled berotralstat at 2-times the maximum recommended dosage of 150 mg, berotralstat represented 34% of the total plasma radioactivity, with 8 metabolites, each accounting for between 1.8 and 7.8% of the total radioactivity.
Specific Populations
In adults and pediatric patients 12 to <18 years of age, no clinically significant differences in the pharmacokinetics of ORLADEYO were observed based on age (12 to 74 years), sex, race, and body weight.
Pediatric Patients
Based on population pharmacokinetic analyses that included pediatric patients 12 to <18 years of age, exposure at steady state following oral administration of berotralstat 150 mg once daily was approximately 20% higher compared to adults. The higher exposure in pediatric patients 12 to <18 years of age is not considered to be clinically significant.
Based on population pharmacokinetics analyses that included pediatric patients 2 to <12 years of age, body weight was the only covariate with a clinically significant impact on the systemic exposure of berotralstat. In pediatric patients 2 to <12 years of age at the recommended dosage based on body weight, median Cmax at steady state is expected to be up to 24% higher compared to adults. However, this difference is not considered to be clinically significant [see Dosage and Administration (2.2) and Use in Special Populations (8.4)].
Patients with Renal Impairment
The pharmacokinetics of a single oral dose of berotralstat at 1.33-times the highest recommended dosage of 150 mg were studied in adult subjects with severe renal impairment (CLCR less than 30 mL/min, estimated by Cockcroft-Gault). When compared to a concurrent cohort with normal renal function (CLCR greater than 90 mL/min, estimated by Cockcroft-Gault), no clinically significant difference was observed; Cmax was increased by 47%, while AUC0-last was increased by 14% [see Use in Specific Populations (8.6)].
The pharmacokinetics of berotralstat has not been studied in patients with End-Stage Renal Disease (CLCR less than 15 mL/min or eGFR less than 15 mL/min/1.73 m2 or patients requiring hemodialysis).
Patients with Hepatic Impairment
The pharmacokinetics of a single 150 mg oral dose of berotralstat were studied in adult subjects with mild, moderate, and severe hepatic function (Child-Pugh Class A, B, and C, respectively). The pharmacokinetics of berotralstat were unchanged in subjects with mild hepatic impairment compared to subjects with normal hepatic function. In subjects with moderate hepatic impairment, Cmax was increased by 77%, while AUC0-inf was increased by 78%. In subjects with severe hepatic impairment, Cmax was increased by 27%, while AUC0-last was decreased by 5%. The median half-life of berotralstat was increased by 37% and 22% in patients with moderate and severe hepatic impairment, respectively, in comparison to healthy subjects. The percent of unbound berotralstat increased 2-fold from a mean of 1.2% in healthy subjects to a mean of 2.4% in subjects with severe hepatic impairment [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Effect of Other Drugs on the Pharmacokinetics of ORLADEYO
Berotralstat is a P-gp and BCRP substrate. Cyclosporine, a P-gp and BCRP inhibitor, decreased berotralstat 150 mg Cmax by 7%, while AUC0-last and AUC0-inf increased by 27% and 24%, respectively.
Effect of ORLADEYO on the Pharmacokinetics of Other Drugs
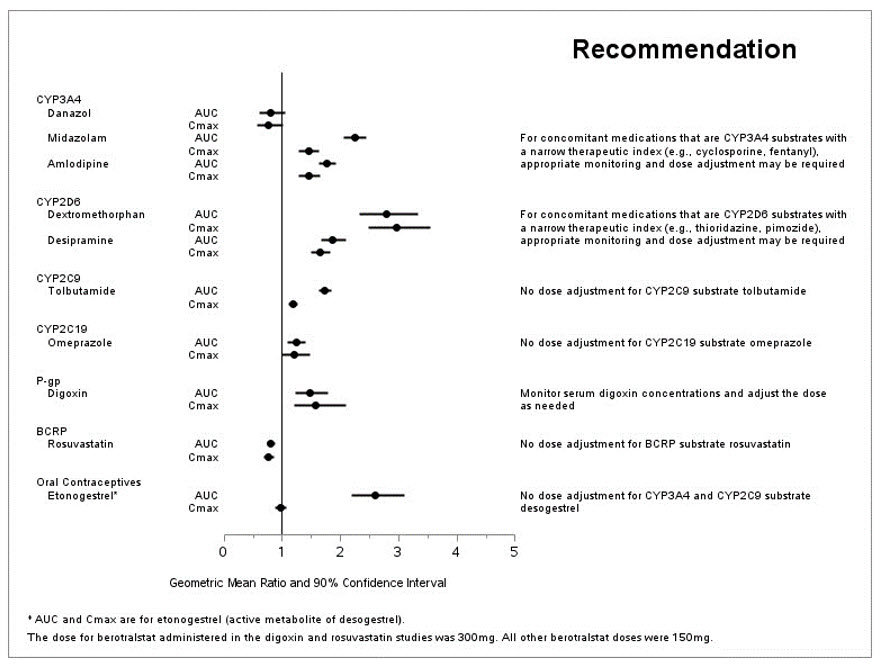
Berotralstat 150 mg once daily is a moderate inhibitor of CYP2D6 and CYP3A4, and a weak inhibitor of CYP2C9 and CYP2C19.
At 2-times the maximum recommended dosage of 150 mg, berotralstat is an inhibitor of P-gp and is not an inhibitor of BCRP (rosuvastatin exposure was decreased by approximately 20%).
In a drug interaction study conducted in healthy females of reproductive potential (N=22), concomitant administration of berotralstat 150 mg once daily with 0.15 mg/0.03 mg desogestrel/ethinyl estradiol led to a 2.6-fold increase in the AUC0-last of etonogestrel, the active metabolite of desogestrel.
The effect of berotralstat on the pharmacokinetics of other drugs are presented in Figure 1 [see Drug Interactions (7.2)].
Figure 1: Effect of ORLADEYO on Concomitant Medications
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity of berotralstat was evaluated in a 2-year study in Wistar rats and a 26-week study in Tg.rasH2 transgenic mice. The berotralstat doses (oral gavage) were up to 20 and 50 mg/kg/day in rats and mice (approximately 5- and 10-times the MRHDD on a plasma AUC basis, respectively). No evidence of tumorigenicity was observed in either species.
-
14 CLINICAL STUDIES
The efficacy of ORLADEYO for the prevention of angioedema attacks in adult and pediatric patients 12 years of age and older with Type I or II HAE was demonstrated in Part 1 of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial (Trial 1 [NCT3485911]).
The trial included 120 adults and pediatric patients 12 to <18 years of age who experienced at least two investigator-confirmed attacks within the first 8 weeks of the run-in period and took at least one dose of study treatment. Patients were randomized into 1 of 3 parallel treatment arms, stratified by baseline attack rate, in a 1:1:1 ratio (ORLADEYO 110 mg, ORLADEYO 150 mg, or placebo by oral administration once daily, with food) for the 24-week treatment period (Part 1).
Patients discontinued other prophylactic HAE medications prior to entering the trial; however, all patients were allowed to use rescue medications for treatment of breakthrough HAE attacks.
A history of laryngeal angioedema attacks was reported in 74% of patients and 75% reported prior use of long-term prophylaxis. The median attack rate during the prospective run-in period (baseline attack rate) was 2.9/month. Seventy percent of patients enrolled had a baseline attack rate of ≥2 attacks/month.
ORLADEYO 150 mg and 110 mg produced statistically significant reductions in the rate of HAE attacks compared to placebo for the primary endpoint in the Intent-to-Treat (ITT) population as shown in Table 3. The percent reductions in HAE attack rate were greater with ORLADEYO 150 mg and 110 mg relative to placebo, regardless of attack rate during the run-in period.
Table 3. Primary Efficacy Endpoint (Trial 1): Reduction in HAE Attack Rate - ITT Population (Adult and Pediatric Patients 12 Years of Age and Older) Outcome ORLADEYO Placebo 110 mg QD 150 mg QD N = 41 N = 40 N = 40* - *
- One patient in the ITT analysis was randomized to placebo but was not treated.
- †
- Statistical analysis based on a negative binomial regression model; number of attacks included as dependent variable, treatment included as fixed effect, baseline attack rate included as covariate, and logarithm of duration on treatment included as offset variable.
- ‡
- Percent reduction relative to placebo.
HAE Attack Rate, rate per 28 days † 1.65 1.31 2.35 % Rate Reduction ‡ (95% CI) 30.0%
(4.6, 48.7)44.2%
(23.0, 59.5)- p-value 0.024 <0.001 - Reductions in attack rates were observed in the first month of treatment with ORLADEYO 150 mg and 110 mg and were sustained through 24 weeks as shown in Figure 2.
Figure 2. Mean (+/- SEM) HAE Attack Rate/Month Through 24 Weeks (Trial 1) - ITT Population (Adult and Pediatric Patients 12 Years of Age and Older)
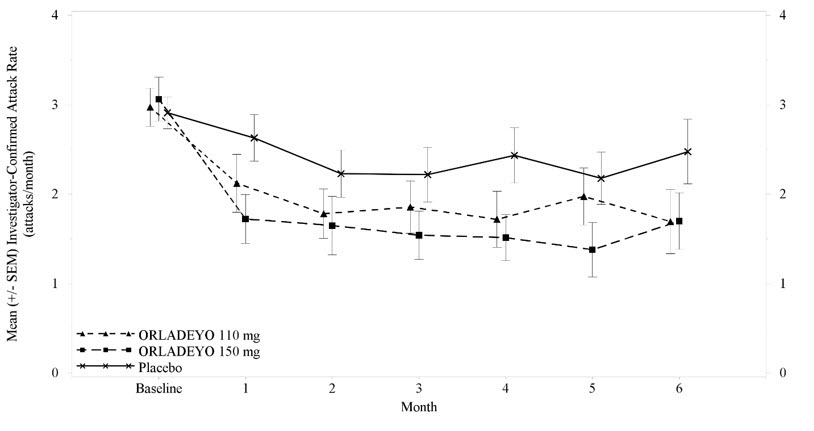
Pre-defined exploratory endpoints included the proportion of responders to ORLADEYO, defined as at least a 50% relative reduction in HAE attacks during treatment compared with the baseline attack rate; 58% of patients who received ORLADEYO 150 mg and 51% of patients who received ORLADEYO 110 mg had a ≥50% reduction in their HAE attack rates compared to baseline versus 25% of placebo patients. In post hoc analyses, 50% and 23% of patients who received ORLADEYO 150 mg, and 27% and 10% of patients who received ORLADEYO 110 mg, had a ≥70% or ≥90% reduction in their HAE attack rates compared to baseline versus 15% and 8% of placebo-treated patients, respectively. The rate of HAE attacks rated as moderate or severe was reduced by 40% and 10% in patients who received ORLADEYO 150 mg and 110 mg, respectively, versus placebo-treated patients.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ORLADEYO (berotralstat) capsules and oral pellets are supplied as follows in Table 4:
Table 4. Dosage Form, Strength, and Package Configuration for ORLADEYO Dosage Form Strength Description Package Configuration NDC Capsule 110 mg light blue opaque body and cap with a white imprint "110" on body and a white imprint "BCX" on cap A 28-day supply of ORLADEYO is provided in a carton containing 4 child-resistant shellpacks, each containing a 7-capsule blister card. Each carton contains a tamper-evident seal. Do not use if tamper-evident seal is broken or missing. 72769-102-01 150 mg white opaque body with a black imprint "150" and a light blue opaque cap with a black imprint "BCX 72769-101-01 Pellets 72 mg white to off-white, film-coated pellets A 28-day supply of ORLADEYO oral pellets is provided in a carton containing 4 wallets, each containing 7 child-resistant unit-dose packets. Each carton contains a tamper-evident seal. Do not use if tamper-evident seal is broken or missing. 72769-111-02 96 mg 72769-112-02 108 mg 72769-113-02 132 mg 72769-114-02 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the risks and benefits of ORLADEYO before prescribing or administering to the patient.
Not for Acute Treatment of HAE Attacks
Advise patients to take their usual rescue medication to treat an acute attack of HAE. Inform patients that the safety and effectiveness of ORLADEYO has not been established as a treatment for acute HAE attacks [see Indications and Usage (1)].
QTc Prolongation with Higher Than Recommended Dosages
Advise patients that they should not take daily doses higher than their prescribed once-daily dose or additional doses of ORLADEYO to treat an acute attack of HAE due to risk of QTc interval prolongation [see Indications and Usage (1) and Warnings and Precautions (5.1)].
Administration Instructions for Oral Pellets
Instruct patients that ORLADEYO oral pellets should not be chewed or crushed because this will affect the film coating, which masks the bitter taste. Advise patients to take ORLADEYO oral pellets by pouring one packet directly into the mouth and swallowing with a non-acidic liquid (e.g., water or milk), or to sprinkle one packet over one tablespoon of soft, non-acidic food and consume immediately [see Dosage and Administration (2.5)].
Drug Interactions
Advise patients that ORLADEYO may interact with other drugs [see Drug Interactions (7) and Clinical Pharmacology (12.3)]. Advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION ORLADEYO® (or-luh-DAY-oh)
(berotralstat)
capsules, for oral useORLADEYO® (or-luh-DAY-oh)
(berotralstat)
oral pelletsThis Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 12/2025 What is ORLADEYO? - ORLADEYO is a prescription medicine used to prevent attacks of Hereditary Angioedema (HAE) in adults and children 2 years of age and older.
- ORLADEYO is not used to treat an acute HAE attack.
- Do not take more than 1 dose of ORLADEYO a day because extra doses can cause heart rhythm problems.
- It is not known if ORLADEYO is safe and effective to treat an acute HAE attack.
- It is not known if ORLADEYO is safe and effective in children under 2 years of age.
Before you take ORLADEYO, tell your healthcare provider about all of your medical conditions, including if you: - have liver problems or kidney problems.
- are pregnant or planning to become pregnant. It is not known if ORLADEYO can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ORLADEYO passes into your breastmilk. Talk to your healthcare provider about the best way to feed your baby while taking ORLADEYO.
Taking ORLADEYO with certain other medicines may affect the way other medicines work and other medicines may affect how ORLADEYO works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How should I take ORLADEYO? - Take ORLADEYO exactly as your healthcare provider tells you to take it.
- Take ORLADEYO with food.
- ORLADEYO capsules (for adults and children ages 12 years and older): Take 1 capsule, by mouth, 1 time every day.
- ORLADEYO oral pellets (ages 2 years to under 12 years): Take 1 packet, by mouth, 1 time every day.
- Eat a meal either right before or right after you take your dose.
- Do not chew or crush the oral pellets because this will affect the film coating and result in bitter taste.
- Hold the packet with the cut line on top.
- Gently tap the packet to settle the oral pellets.
- Open the packet along the cut line.
- Take ORLADEYO oral pellets in 1 of the following ways:
- Pour all the oral pellets in the packet directly into the mouth and swallow right away with a non-acidic liquid such as water or milk.
or - Carefully sprinkle all the oral pellets in the packet over 1 tablespoon (about 15mL) of soft, non-acidic food and take right away. Food should be at or below room temperature.
Examples of soft, non-acidic foods include pudding, mashed potatoes, creamed corn, pureed peas, pureed bananas, and pureed carrots.
- Pour all the oral pellets in the packet directly into the mouth and swallow right away with a non-acidic liquid such as water or milk.
- To prevent the taste-masking coating from dissolving and resulting in a bitter taste:
- Take the oral pellets within 10 minutes of being sprinkled over food. Throw the oral pellets away if not taken within 10 minutes of sprinkling over food.
- Do not sprinkle the oral pellets on acidic foods such as yogurt and applesauce.
What are the possible side effects of ORLADEYO?
Taking more than 1 dose of ORLADEYO a day may cause serious side effects, including:- heart rhythm problems. A heart rhythm problem called QTc interval prolongation can happen in people who take more than 1 dose of ORLADEYO a day. This condition can cause an abnormal heart beat. Do not take more than 1 dose of ORLADEYO a day.
- abdominal pain
- vomiting
- diarrhea
- back pain
- heartburn
Less common side effects include increases in liver function tests. Rarely, some people had a brief, itchy rash.
These are not all of the possible side effects of ORLADEYO. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ORLADEYO? - Store ORLADEYO at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Each carton contains a tamper evident seal. Do not use ORLADEYO if the tamper evident seal is broken or missing.
General information about the safe and effective use of ORLADEYO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ORLADEYO for a condition for which it was not prescribed. Do not give ORLADEYO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ORLADEYO that is written for health professionals.What are the ingredients in ORLADEYO?
ORLADEYO capsules:
Active ingredient: berotralstat dihydrochloride
Inactive ingredients: colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starchORLADEYO oral pellets:
Active ingredient: berotralstat dihydrochloride
Inactive ingredients: colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starch.
The oral pellets film-coating contains: butylated methacrylate copolymer, silicon dioxide, sodium lauryl sulphate, stearic acid, talc, and titanium dioxide.Manufactured for: BioCryst Pharmaceuticals, Inc., Durham, NC 27703
ORLADEYO® is a registered trademark of BioCryst Pharmaceuticals, Inc.
© 2025 BioCryst. All rights reserved.
ORL:2PPI
For more information, visit www.ORLADEYO.com or call 1-833-633-2279. - PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Card Shellpack Carton
- PRINCIPAL DISPLAY PANEL - 110 mg Capsule Blister Card Shellpack Carton
- PRINCIPAL DISPLAY PANEL - 72 mg Pellet Packet Wallet Carton
- PRINCIPAL DISPLAY PANEL - 96 mg Pellet Packet Wallet Carton
- PRINCIPAL DISPLAY PANEL - 108 mg Pellet Packet Wallet Carton
- PRINCIPAL DISPLAY PANEL - 132 mg Pellet Packet Wallet Carton
-
INGREDIENTS AND APPEARANCE
ORLADEYO
berotralstat hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72769-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Berotralstat hydrochloride (UNII: 88SH1NBL2B) (Berotralstat - UNII:XZA0KB1BDQ) Berotralstat 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) Magnesium Stearate (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE, BLUE (light blue) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 150;BCX Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72769-101-01 4 in 1 CARTON 12/04/2020 1 1 in 1 DOSE PACK 1 7 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214094 12/04/2020 ORLADEYO
berotralstat hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72769-102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Berotralstat hydrochloride (UNII: 88SH1NBL2B) (Berotralstat - UNII:XZA0KB1BDQ) Berotralstat 110 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) Magnesium Stearate (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color BLUE (light blue) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 110;BCX Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72769-102-01 4 in 1 CARTON 12/04/2020 1 1 in 1 DOSE PACK 1 7 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214094 12/04/2020 ORLADEYO
berotralstat hydrochloride pelletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72769-111 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Berotralstat hydrochloride (UNII: 88SH1NBL2B) (Berotralstat - UNII:XZA0KB1BDQ) Berotralstat 72 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) Magnesium Stearate (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Stearic acid (UNII: 4ELV7Z65AP) Talc (UNII: 7SEV7J4R1U) Titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72769-111-02 4 in 1 CARTON 12/12/2020 1 7 in 1 DOSE PACK 1 12 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219776 12/04/2020 ORLADEYO
berotralstat hydrochloride pelletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72769-112 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Berotralstat hydrochloride (UNII: 88SH1NBL2B) (Berotralstat - UNII:XZA0KB1BDQ) Berotralstat 96 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) Magnesium Stearate (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Stearic acid (UNII: 4ELV7Z65AP) Talc (UNII: 7SEV7J4R1U) Titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72769-112-02 4 in 1 CARTON 12/12/2020 1 7 in 1 DOSE PACK 1 16 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219776 12/04/2020 ORLADEYO
berotralstat hydrochloride pelletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72769-113 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Berotralstat hydrochloride (UNII: 88SH1NBL2B) (Berotralstat - UNII:XZA0KB1BDQ) Berotralstat 108 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) Magnesium Stearate (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Stearic acid (UNII: 4ELV7Z65AP) Talc (UNII: 7SEV7J4R1U) Titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72769-113-02 4 in 1 CARTON 12/12/2020 1 7 in 1 DOSE PACK 1 18 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219776 12/04/2020 ORLADEYO
berotralstat hydrochloride pelletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72769-114 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Berotralstat hydrochloride (UNII: 88SH1NBL2B) (Berotralstat - UNII:XZA0KB1BDQ) Berotralstat 132 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) Magnesium Stearate (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Stearic acid (UNII: 4ELV7Z65AP) Talc (UNII: 7SEV7J4R1U) Titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72769-114-02 4 in 1 CARTON 12/12/2020 1 7 in 1 DOSE PACK 1 22 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA219776 12/04/2020 Labeler - BioCryst Pharmaceuticals Inc. (618194609) Establishment Name Address ID/FEI Business Operations Cambrex Charles City 782974257 API MANUFACTURE(72769-101, 72769-102) , ANALYSIS(72769-101, 72769-102) Establishment Name Address ID/FEI Business Operations Catalent CTS, LLC 962674474 MANUFACTURE(72769-101, 72769-102) , ANALYSIS(72769-101, 72769-102) Establishment Name Address ID/FEI Business Operations Catalent Pharma Solutions LLC 014904112 PACK(72769-101, 72769-102) Establishment Name Address ID/FEI Business Operations Patheon Pharmaceuticals, Inc. 005286822 MANUFACTURE(72769-101, 72769-102) , ANALYSIS(72769-101, 72769-102) Establishment Name Address ID/FEI Business Operations AndersonBrecon Inc. A PCI Pharma Services Company 053217022 PACK(72769-101, 72769-102) , LABEL(72769-101, 72769-102) Establishment Name Address ID/FEI Business Operations Avista Pharma Solutions, Inc. dba Cambrex 108353231 ANALYSIS(72769-101, 72769-102, 72769-111, 72769-112, 72769-113, 72769-114) Establishment Name Address ID/FEI Business Operations Quotient Sciences - Philadelphia, LLC 126874135 ANALYSIS(72769-111, 72769-112, 72769-113, 72769-114) , MANUFACTURE(72769-111, 72769-112, 72769-113, 72769-114) Establishment Name Address ID/FEI Business Operations Halo Pharmaceutical Inc 829609168 LABEL(72769-111, 72769-112, 72769-113, 72769-114) , PACK(72769-111, 72769-112, 72769-113, 72769-114)












