FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ORLADEYO® is indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and pediatric patients 12 years of age and older.
Limitations of Use:
The safety and effectiveness of ORLADEYO for the treatment of acute HAE attacks have not been established. ORLADEYO should not be used for treatment of acute HAE attacks. Additional doses or doses of ORLADEYO higher than 150 mg once daily are not recommended due to the potential for QT prolongation [see Warnings and Precautions (5.1)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of ORLADEYO is one 150 mg capsule taken orally once daily with food.
2.2 Recommended Dosage in Patients with Hepatic Impairment
No dosage adjustment of ORLADEYO is recommended for patients with mild hepatic impairment (Child-Pugh Class A) [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
In patients with moderate or severe hepatic impairment (Child-Pugh B or C), the recommended dosage of ORLADEYO is one 110 mg capsule taken orally once daily with food [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.3 Dosage Adjustment in Patients with Persistent GI Reactions
Gastrointestinal (GI) reactions may occur in patients receiving ORLADEYO [see Adverse Reactions (6.1)]. If GI events persist, a reduced dose of 110 mg once daily with food may be considered.
5 WARNINGS AND PRECAUTIONS
5.1 Risk of QT Prolongation with Higher-Than-Recommended Dosages
ORLADEYO should not be used for treatment of acute attacks of HAE. Additional doses or doses of ORLADEYO higher than 150 mg once daily are not recommended. An increase in QT was observed at dosages higher than the recommended 150 mg once daily dosage and was concentration dependent [see Clinical Pharmacology (12.2)].
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
- QT Prolongation [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ORLADEYO is primarily based on 24-week (Part 1) data from a 3-part, double-blind, parallel-group, placebo-controlled study (Trial 1) in 120 patients with Type I or II HAE randomized and dosed with either ORLADEYO 110 mg, 150 mg, or placebo, once daily with food. After Week 24, patients who continued in the study received active treatment through 48 weeks.
In Trial 1, a total of 81 patients aged 12 years and older with HAE received at least one dose of ORLADEYO in Part 1. Overall, 66% of patients were female and 93% of patients were Caucasian with a mean age of 41.6 years. The proportion of patients who discontinued study drug prematurely due to adverse reactions was 7% and 3% for patients treated with 110 mg and 150 mg ORLADEYO, respectively, and 3% for placebo-treated patients. No deaths occurred in the trial.
The safety profile of ORLADEYO was generally similar across all subgroups of patients, including analysis by age, sex, and geographic region.
Table 1 shows adverse reactions occurring in ≥10% of patients in any ORLADEYO treatment group that also occurred at a higher rate than in the placebo treatment group in Trial 1.
| Adverse Reaction | Placebo (N=39) | ORLADEYO | ||
|---|---|---|---|---|
| 110 mg (N=41) | 150 mg (N=40) | Total (N=81) |
||
| n (%) | n (%) | n (%) | n (%) | |
| Abdominal Pain* | 4 (10) | 4 (10) | 9 (23) | 13 (16) |
| Vomiting | 1 (3) | 4 (10) | 6 (15) | 10 (12) |
| Diarrhea† | 0 | 4 (10) | 6 (15) | 10 (12) |
| Back Pain | 1 (3) | 1 (2) | 4 (10) | 5 (6) |
| Gastroesophageal Reflux Disease | 0 | 4 (10) | 2 (5) | 6 (7) |
Gastrointestinal reactions, including abdominal pain, vomiting, and diarrhea occurred more frequently in patients receiving ORLADEYO 150 mg versus ORLADEYO 110 mg or placebo. These reactions generally occurred early after initiation of treatment with ORLADEYO, became less frequent with time, and typically self-resolved. No patients in the ORLADEYO 150 mg dose group and 1 patient in the ORLADEYO 110 mg dose group discontinued treatment due to a gastrointestinal adverse reaction.
Less Common Adverse Reactions
Other adverse reactions that occurred in Part 1 of Trial 1 with an incidence between 5% and <10% at a higher incidence in ORLADEYO-treated patients compared to placebo included headache (9% versus 5%), fatigue (6% versus 3%), and flatulence (6% versus 3%).
A maculopapular drug rash was reported in less than 1% of patients treated with ORLADEYO. The rash resolved, including in subjects who continued dosing.
Safety data are also available from 227 patients enrolled in an ongoing, open-label, long-term safety study (Trial 2) who received ORLADEYO 110 mg (N=100) or 150 mg (N=127) once daily with food and are consistent with the 24-week controlled safety data from Trial 1 (Part 1).
Laboratory Abnormalities
Transaminase elevations
In Part 1 of Trial 1, a single 150 mg ORLADEYO-treated patient discontinued treatment due to asymptomatic elevated transaminases (ALT >8× the upper limit of normal [ULN] and AST >3× ULN). Total bilirubin was normal. No subject receiving 110 mg or placebo developed transaminase levels >3× ULN. In addition to this patient, 2 ORLADEYO-treated patients developed laboratory-related hepatic adverse events compared to 1 placebo-treated patient. No patient reported serious adverse reactions of elevated transaminases.
7 DRUG INTERACTIONS
This section describes clinically relevant drug interactions with ORLADEYO. Drug interaction studies are described elsewhere in the labeling [see Clinical Pharmacology (12.3)].
7.2 Potential for ORLADEYO to Affect Other Drugs
CYP2D6 and CYP3A4 Substrates
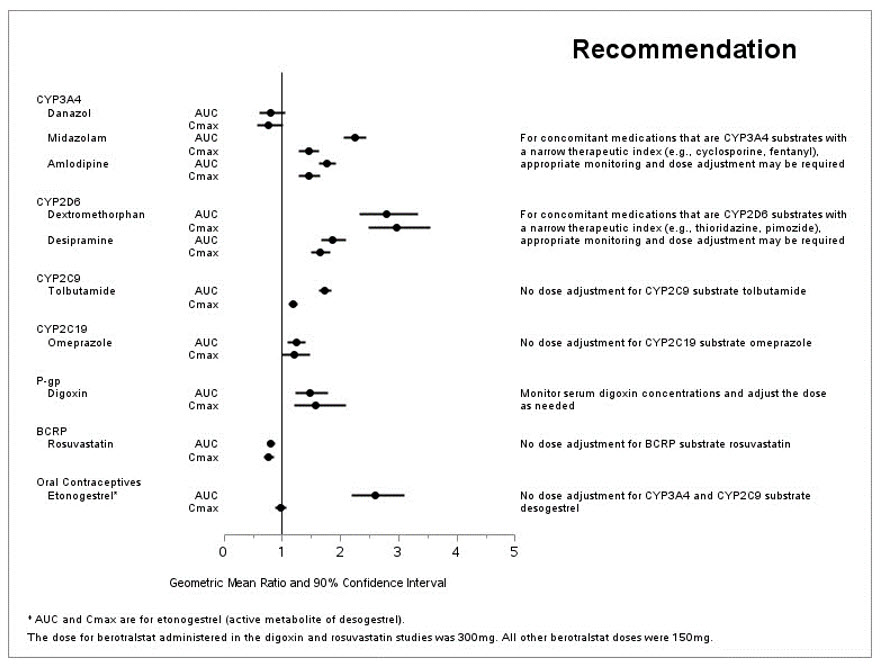
ORLADEYO at a dose of 150 mg is a moderate inhibitor of CYP2D6 and CYP3A4. For concomitant medications with a narrow therapeutic index that are predominantly metabolized by CYP2D6 (e.g., thioridazine, pimozide) or CYP3A4 (e.g., cyclosporine, fentanyl), appropriate monitoring and dose titration is recommended [see Clinical Pharmacology (12.3)].
Desogestrel
In a drug interaction study conducted in healthy women of childbearing potential (N=22), co-administration of ORLADEYO at a dose of 150 mg at steady state with a single dose of 0.15 mg/0.03 mg desogestrel/ethinyl estradiol resulted in increased exposure to etonogestrel, the active metabolite of desogestrel. If ORLADEYO is co-administered with desogestrel, any potential risk of concurrent use of desogestrel should be weighed against benefit [see Clinical Pharmacology (12.3)].
P-gp Substrates
ORLADEYO at a dose of 300 mg is a P-gp inhibitor. Appropriate monitoring and dose titration is recommended for P-gp substrates (e.g., digoxin) when co-administering with ORLADEYO [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient data in pregnant women available to inform drug-related risks with ORLADEYO use in pregnancy. Based on animal reproduction studies, no evidence of structural alterations was observed when berotralstat was administered orally to pregnant rats and rabbits during organogenesis at doses up to approximately 10 and 2 times, respectively, the maximum recommended human daily dose (MRHDD) in adults on an AUC basis (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In animal reproduction studies, oral administration of berotralstat to pregnant rats and rabbits during the period of organogenesis did not cause fetal structural alterations. The berotralstat dose in rats and rabbits was up to approximately 10 and 2 times, respectively, the MRHDD in adults (on an AUC basis at maternal doses of 75 and 100 mg/kg/day, respectively). In a pre- and postnatal development study in rats, oral administration of berotralstat to pregnant rats during the period of organogenesis and until delivery at doses up to 45 mg/kg/day (approximately 2 times of the MRHDD on a mg/m2 basis) did not cause fetal structural alterations either. Berotralstat concentrations in the fetal blood were approximately 5-11% of the maternal blood.
8.2 Lactation
Risk Summary
There are no data on the presence of berotralstat in human milk, its effects on the breastfed infant, or its effects on milk production. However, when a drug is present in animal milk, it is likely that the drug will be present in human milk. Low levels of berotralstat were detected in the plasma of rat pups when dams were dosed with the drug orally during the lactation period. The berotralstat concentration in the pup plasma was approximately 2% of the maternal plasma (see Data).
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ORLADEYO and any potential adverse effects on the breastfed infant from ORLADEYO or from the underlying maternal condition.
Data
Animal Data
In the pre- and post-natal development study in rats, berotralstat was administered to dams during the pregnancy and lactation periods at doses up to 45 mg/kg/day (approximately 2 times of the MRHDD on a mg/m2 basis). Berotralstat was detected in the plasma of pups during the lactation period. The berotralstat concentration in the pup plasma was approximately 2% of the maternal plasma. Both dams and pups at 45 mg/kg/day showed statistically significant decreases in body weight gain (p<0.05). No treatment-related effects were observed at 25 mg/kg/day (approximately equal to the MRHDD on a mg/m2 basis).
8.4 Pediatric Use
The safety and effectiveness of ORLADEYO for prophylaxis to prevent attacks of hereditary angioedema have been established in pediatric patients aged 12 and older. Use of ORLADEYO in this population is supported by evidence from an adequate and well-controlled study (Trial 1) that included adults and a total of 6 adolescent patients aged 12 to <18 years of age. The safety profile and attack rate on study were similar to those observed in adults [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)]. An additional 10 adolescent patients aged 12 to <18 years were enrolled in the open-label study (Trial 2).
The safety and effectiveness of ORLADEYO in pediatric patients <12 years of age have not been established.
8.5 Geriatric Use
The safety and effectiveness of ORLADEYO were evaluated in a subgroup of patients (N=9) aged ≥65 years in Trial 1. Results of the subgroup analysis by age were consistent with overall study results. The safety profile from an additional 5 elderly patients aged ≥65 years enrolled in the open-label, long-term safety study (Trial 2) was consistent with data from Trial 1 [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
8.6 Renal Impairment
No dosage adjustment of ORLADEYO is recommended for patients with mild, moderate, or severe renal impairment [see Clinical Pharmacology (12.3)].
ORLADEYO has not been studied in patients with End-Stage Renal Disease (CLCR <15 mL/min or eGFR <15 mL/min/1.73 m2 or patients requiring hemodialysis), and therefore is not recommended for use in these patient populations [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment of ORLADEYO is recommended for patients with mild hepatic impairment (Child-Pugh Class A) [see Clinical Pharmacology (12.3)].
In patients with moderate or severe hepatic impairment (Child-Pugh B or C), the recommended dose of ORLADEYO is 110 mg once daily with food [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
11 DESCRIPTION
ORLADEYO (berotralstat) capsules is a plasma kallikrein inhibitor. Berotralstat is presented as the dihydrochloride salt with the chemical name 1-[3-(aminomethyl)phenyl]-N-(5-{(R)-(3-cyanophenyl)[(cyclopropylmethyl)amino]methyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide dihydrochloride. The chemical structure is:
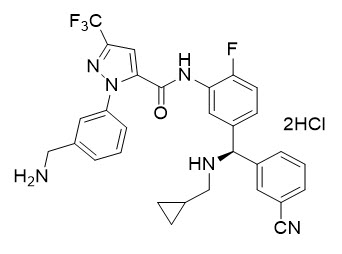
Berotralstat dihydrochloride is a white to off-white powder that is soluble in water at pH ≤4. The molecular formula is C30H26F4N6O ∙ 2HCl and the molecular weight is 635.49 (dihydrochloride).
ORLADEYO is supplied as 150 mg (equivalent to 169.4 mg berotralstat dihydrochloride) and 110 mg (equivalent to 124.3 mg berotralstat dihydrochloride) hard gelatin capsules for oral administration. Each capsule contains the active ingredient berotralstat dihydrochloride and the inactive ingredients colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starch.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Berotralstat is a plasma kallikrein inhibitor that binds to plasma kallikrein and inhibits its proteolytic activity. Plasma kallikrein is a protease that cleaves high-molecular-weight kininogen (HMWK) to generate cleaved HMWK (cHMWK) and bradykinin, a potent vasodilator that increases vascular permeability resulting in swelling and pain associated with HAE. In patients with HAE due to C1-inhibitor (C1-INH) deficiency or dysfunction, normal regulation of plasma kallikrein activity is not present, which leads to uncontrolled increases in plasma kallikrein activity and results in angioedema attacks. Berotralstat decreases plasma kallikrein activity to control excess bradykinin generation in patients with HAE.
12.2 Pharmacodynamics
Concentration-dependent inhibition of plasma kallikrein, measured as a reduction from baseline of specific enzyme activity, was demonstrated after oral administration of ORLADEYO once daily in patients with HAE.
Cardiac Electrophysiology
At the recommended dose of 150 mg once daily, ORLADEYO does not prolong the QT interval to any clinically relevant extent. At 3 times the recommended dose, the mean (upper 90% confidence interval) increase in QTcF was 15.9 msec (23.5 msec). The observed increase in QTcF was concentration-dependent.
12.3 Pharmacokinetics
Following oral administration of berotralstat 150 mg once daily, the steady-state Cmax and area under the curve over the dosing interval (AUCtau) are 158 ng/mL (range: 110 to 234 ng/mL) and 2770 ng*hr/mL (range: 1880 to 3790 ng*hr/mL), respectively. Following oral administration of berotralstat 110 mg once daily, the steady-state Cmax and AUCtau are 97.8 ng/mL (range: 63 to 235 ng/mL) and 1600 ng*hr/mL (range: 950 to 4170 ng*hr/mL), respectively.
Berotralstat exposure (Cmax and AUC) increases greater than proportionally with dose and steady state is reached by days 6 to 12. After once-daily administration, exposure of berotralstat at steady state is approximately 5 times that after a single dose.
The pharmacokinetics of berotralstat are similar between healthy adult subjects and in patients with HAE.
Absorption
The median time to maximum plasma concentration (Tmax) of berotralstat when administered with food is 5 hours (range: 1 to 8 hours).
Distribution
Plasma protein binding is approximately 99%. After a single dose of radiolabeled berotralstat 300 mg, the blood to plasma ratio was approximately 0.92.
Elimination
The median elimination half-life of berotralstat was approximately 93 hours (range: 39 to 152 hours).
Metabolism
Berotralstat is metabolized by CYP2D6 and by CYP3A4 with low turnover in vitro. After a single oral radiolabeled berotralstat 300 mg dose, berotralstat represented 34% of the total plasma radioactivity, with 8 metabolites, each accounting for between 1.8 and 7.8% of the total radioactivity.
Specific Populations
Body weight, age, gender, and race did not have a clinically meaningful influence on the systemic exposure of berotralstat.
Geriatric Patients
Based on the population pharmacokinetic analyses that included elderly patients (≥65 to 74 years, N=25), age does not have a clinically meaningful impact on the systemic exposure of berotralstat [see Use in Specific Populations (8.5)].
Pediatric Patients
Based on population pharmacokinetic analyses that included pediatric patients 12 to <18 years of age, exposure at steady state following oral administration of berotralstat 150 mg once daily was approximately 20% higher compared to adults. The higher exposure in adolescents is not considered to be clinically meaningful.
Patients with Renal Impairment
The pharmacokinetics of a single 200 mg oral dose of berotralstat were studied in subjects with severe renal impairment (CLCR less than 30 mL/min). When compared to a concurrent cohort with normal renal function (CLCR greater than 90 mL/min), no clinically relevant differences were observed; Cmax was increased by 47%, while AUC0-last was increased by 14% [see Use in Specific Populations (8.6)].
The pharmacokinetics of berotralstat has not been studied in patients with End-Stage Renal Disease (CLCR less than 15 mL/min or eGFR less than 15 mL/min/1.73 m2 or patients requiring hemodialysis).
Patients with Hepatic Impairment
The pharmacokinetics of a single 150 mg oral dose of berotralstat were studied in subjects with mild, moderate, and severe hepatic function (Child-Pugh Class A, B, and C, respectively). The pharmacokinetics of berotralstat were unchanged in subjects with mild hepatic impairment compared to subjects with normal hepatic function. In subjects with moderate hepatic impairment, Cmax was increased by 77%, while AUC0-inf was increased by 78%. In subjects with severe hepatic impairment, Cmax was increased by 27%, while AUC0-last was decreased by 5%. The median half-life of berotralstat was increased by 37% and 22% in patients with moderate and severe hepatic impairment, respectively, in comparison to healthy subjects. The percent of unbound berotralstat increased 2-fold from a mean of 1.2% in healthy subjects to a mean of 2.4% in subjects with severe hepatic impairment [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Effect of Other Drugs on the Pharmacokinetics of ORLADEYO
Berotralstat is a P-gp and BCRP substrate. Cyclosporine, a P-gp and BCRP inhibitor, decreased berotralstat 150 mg Cmax by 7%, while AUC0-last and AUC0-inf increased by 27% and 24%, respectively.
Effect of ORLADEYO on the Pharmacokinetics of Other Drugs
Berotralstat 150 mg once daily is a moderate inhibitor of CYP2D6 and CYP3A4, and a weak inhibitor of CYP2C9 and CYP2C19.
Berotralstat at a 300 mg dose is an inhibitor of P-gp and is not an inhibitor of BCRP (rosuvastatin exposure was decreased by approximately 20%).
Co-administration of berotralstat 150 mg once daily with 0.15 mg/0.03 mg desogestrel/ethinyl estradiol led to a 2.6-fold increase in the AUC0-last of etonogestrel, the active metabolite of desogestrel.
The effect of berotralstat on the pharmacokinetics of other drugs are presented in Figure 1 [see Drug Interactions (7.2)].
Figure 1: Effect of ORLADEYO on Concomitant Medications
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity of berotralstat was evaluated in a 2-year study in Wistar rats and a 26-week study in Tg.rasH2 transgenic mice. The berotralstat doses (oral gavage) were up to 20 and 50 mg/kg/day in rats and mice (approximately 5 and 10 times the MRHDD on a plasma AUC basis, respectively). No evidence of tumorigenicity was observed in either species.
14 CLINICAL STUDIES
Trial 1 (NCT3485911)
The efficacy of ORLADEYO for the prevention of angioedema attacks in patients 12 years of age and older with Type I or II HAE was demonstrated in Part 1 of a multicenter, randomized, double-blind, placebo-controlled, parallel-group study (Trial 1).
The study included 120 adult and adolescent patients who experienced at least two investigator-confirmed attacks within the first 8 weeks of the run-in period and took at least one dose of study treatment. Patients were randomized into 1 of 3 parallel treatment arms, stratified by baseline attack rate, in a 1:1:1 ratio (berotralstat 110 mg, berotralstat 150 mg, or placebo by oral administration once daily, with food) for the 24-week treatment period (Part 1).
Patients discontinued other prophylactic HAE medications prior to entering the study; however, all patients were allowed to use rescue medications for treatment of breakthrough HAE attacks.
A history of laryngeal angioedema attacks was reported in 74% of patients and 75% reported prior use of long-term prophylaxis. The median attack rate during the prospective run-in period (baseline attack rate) was 2.9/month. Seventy percent of patients enrolled had a baseline attack rate of ≥2 attacks/month.
ORLADEYO 150 mg and 110 mg produced statistically significant reductions in the rate of HAE attacks compared to placebo for the primary endpoint in the Intent-to-Treat (ITT) population as shown in Table 2. The percent reductions in HAE attack rate were greater with ORLADEYO 150 mg and 110 mg relative to placebo, regardless of attack rate during the run-in period.
| Outcome | ORLADEYO | Placebo | |
|---|---|---|---|
| 110 mg QD | 150 mg QD | ||
| N = 41 | N = 40 | N = 40* | |
|
|||
| HAE Attack Rate, rate per 28 days † | 1.65 | 1.31 | 2.35 |
| % Rate Reduction ‡ (95% CI) | 30.0% (4.6, 48.7) | 44.2% (23.0, 59.5) | - |
| p-value | 0.024 | <0.001 | - |
Reductions in attack rates were observed in the first month of treatment with ORLADEYO 150 mg and 110 mg and were sustained through 24 weeks as shown in Figure 2.
Figure 2. Mean (+/- SEM) HAE Attack Rate/month Through 24 Weeks (Trial 1)- ITT Population
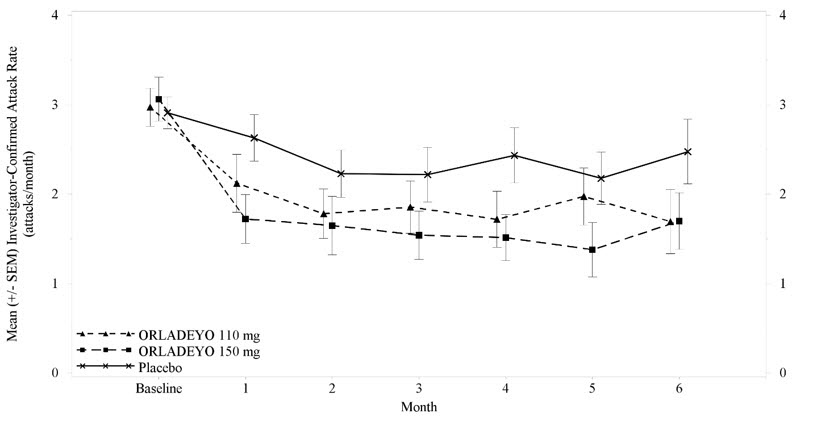
Pre-defined exploratory endpoints included the proportion of responders to study drug, defined as at least a 50% relative reduction in HAE attacks during treatment compared with the baseline attack rate; 58% of patients receiving 150 mg ORLADEYO and 51% of patients receiving 110 mg ORLADEYO had a ≥50% reduction in their HAE attack rates compared to baseline versus 25% of placebo patients. In post hoc analyses, 50% and 23% of patients receiving 150 mg ORLADEYO, and 27% and 10% of patients receiving 110 mg ORLADEYO, had a ≥70% or ≥90% reduction in their HAE attack rates compared to baseline versus 15% and 8% of placebo patients, respectively. The rate of attacks rated as moderate or severe was reduced by 40% and 10% in patients receiving 150 mg ORLADEYO and 110 mg ORLADEYO, respectively, versus placebo.
16 HOW SUPPLIED/STORAGE AND HANDLING
ORLADEYO (berotralstat) capsules:
- 150 mg: a white opaque body with a black imprint "150" and a light blue opaque cap with a black imprint "BCX".
- 110 mg: light blue opaque capsules with a white imprint "110" on body and a white imprint "BCX" on cap.
- A 28-day supply of ORLADEYO is provided in a carton containing four child-resistant shellpaks, each containing a 7-capsule blister card. NDC 72769-101-01 (150 mg) and NDC 72769-102-01 (110 mg).
- Each carton contains a tamper-evident seal.
- Do not use if tamper-evident seal is broken or missing.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the risks and benefits of ORLADEYO before prescribing or administering to the patient.
Drug Interactions
Advise patients that ORLADEYO may interact with other drugs [see Drug Interactions (7) and Clinical Pharmacology (12.3)]. Advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products.
Not for Acute Treatment of HAE Attacks
Advise patients to take their usual rescue medication to treat an acute attack of HAE. Inform patients that the safety and effectiveness of ORLADEYO has not been established as an acute treatment for HAE attacks. Advise patients that they should not take daily doses higher than 150 mg once daily or additional doses of ORLADEYO to treat an acute attack of HAE due to risk of QT prolongation [see Limitations of Use (1) and Warnings and Precautions (5.1)].
For more information, visit www.ORLADEYO.com
ORLADEYO® is a registered trademark of BioCryst Pharmaceuticals, Inc.
Manufactured for:
BioCryst Pharmaceuticals, Inc.
Durham, NC 27703
214094-BC-002
| PATIENT INFORMATION ORLADEYO® (or-luh-DAY-oh) (berotralstat) capsules, for oral use |
|||||
|---|---|---|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. | 03/2022 | ||||
What is ORLADEYO?
|
|||||
Before you take ORLADEYO, tell your healthcare provider about all of your medical conditions, including if you:
Taking ORLADEYO with certain other medicines may affect the way other medicines work and other medicines may affect how ORLADEYO works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|||||
How should I take ORLADEYO?
|
|||||
| What are the possible side effects of ORLADEYO?
Taking more than one capsule of ORLADEYO a day may cause serious side effects, including:
|
|||||
|
|
|
|
|
|
| Less common side effects include increases in liver function tests. Rarely, some patients had a brief, itchy rash. These are not all of the possible side effects of ORLADEYO. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||||
How should I store ORLADEYO?
|
|||||
| General information about the safe and effective use of ORLADEYO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ORLADEYO for a condition for which it was not prescribed. Do not give ORLADEYO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ORLADEYO that is written for health professionals. |
|||||
| What are the ingredients in ORLADEYO?
Active ingredient: berotralstat dihydrochloride Inactive ingredients: colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starch |
|||||
| Manufactured for: BioCryst Pharmaceuticals, Inc., Durham, NC 27703 ORLADEYO® is a registered trademark of BioCryst Pharmaceuticals, Inc. © 2022 BioCryst. All rights reserved. For more information, visit www.ORLADEYO.com or call 1-833-633-2279. |
|||||

