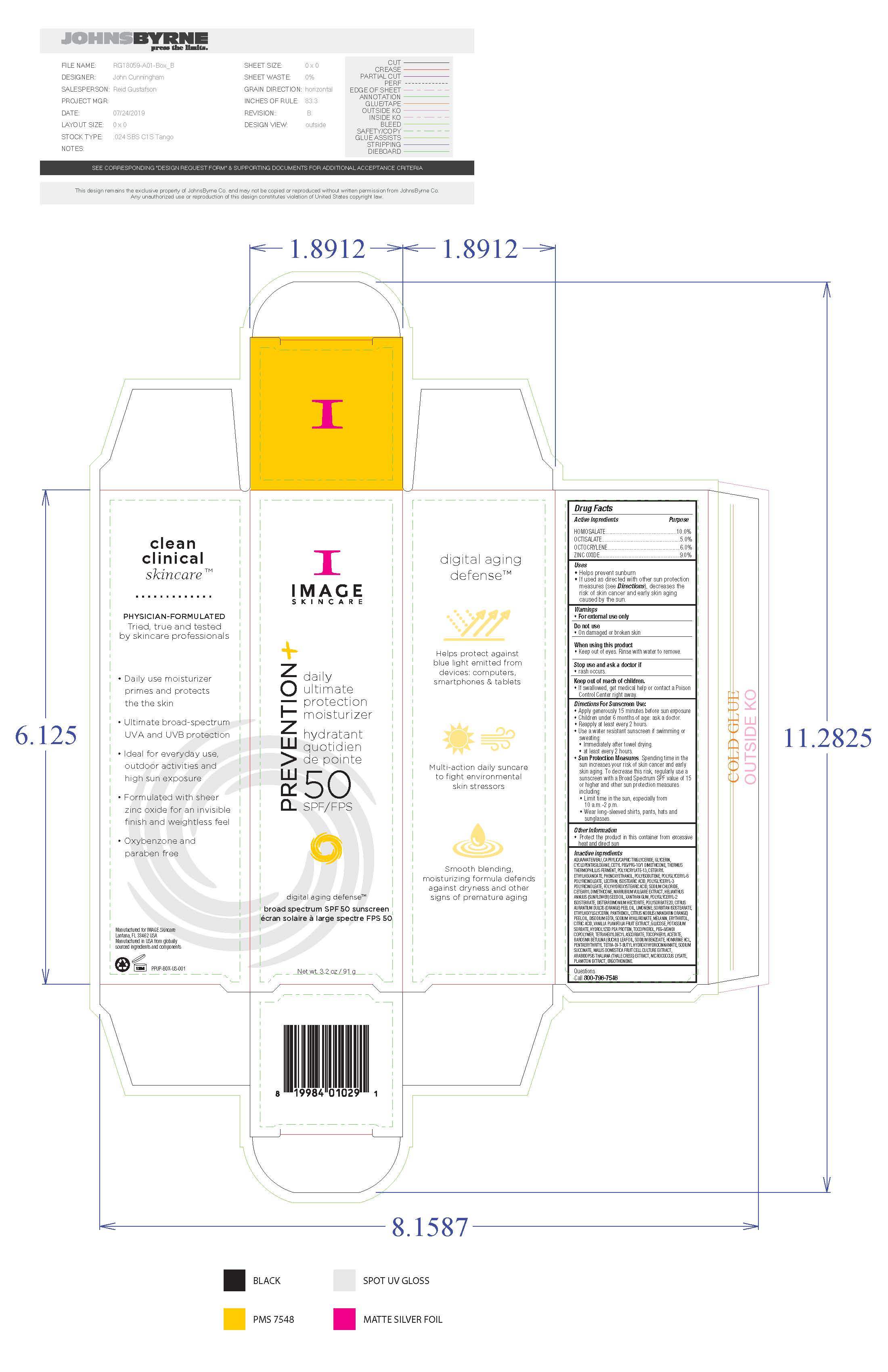
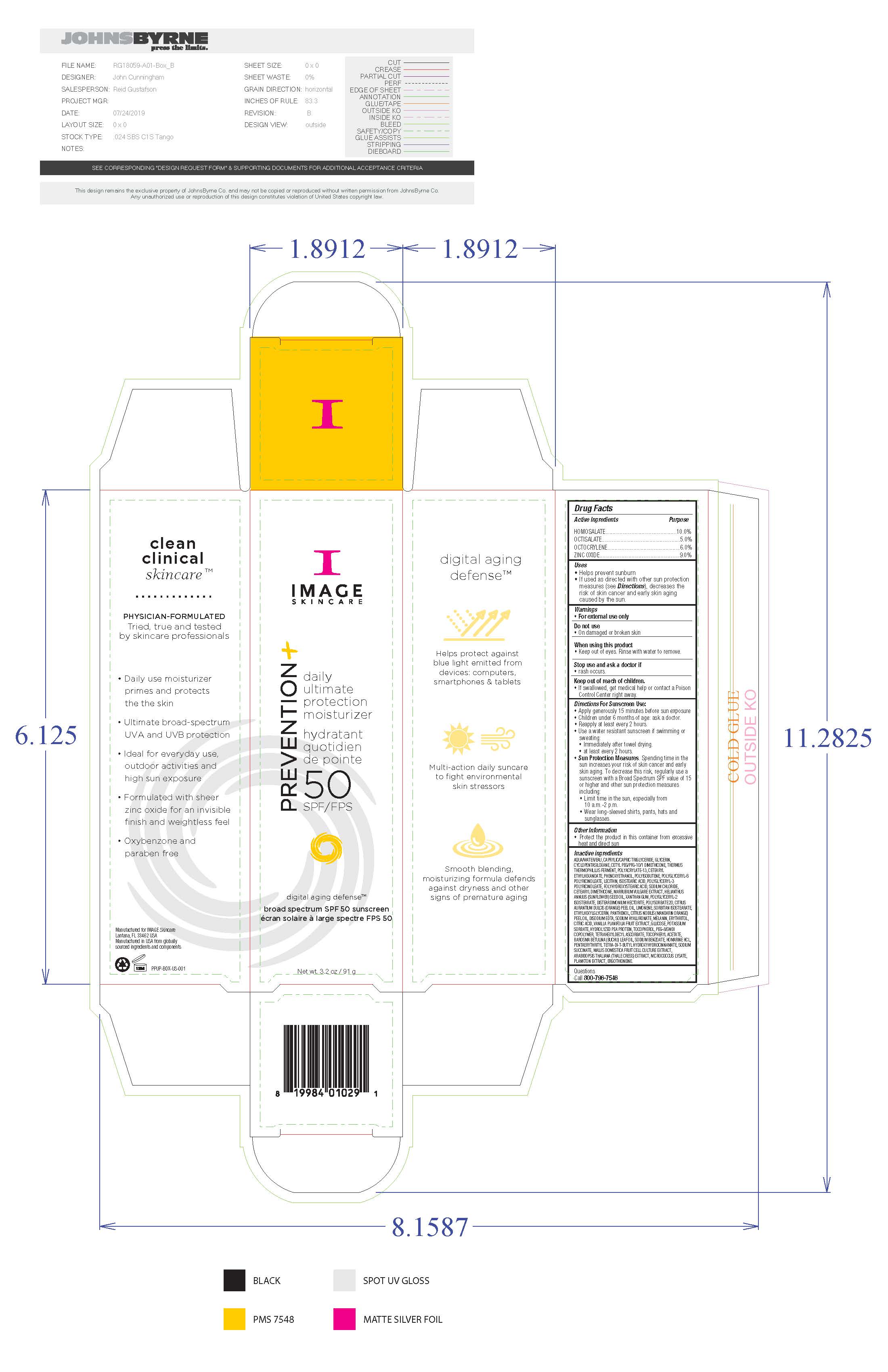
Label: IMAGE SKINCARE PREVENTION PLUS DAILY ULTIMATE PROTECTION- homosalate, octisalate, octocrylene, zinc oxide sunscreen cream
- NDC Code(s): 60232-1022-0, 60232-1022-3
- Packager: Swiss-American CDMO, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Keep out of Reach of Children
- Active Ingredients
- Uses
- Uses
-
Directions for Sunscreen Use
Apply generously 15 minutes before sun exposure. Children under 6 months of age: ask a doctor. Reapply at least every 2 hours. Use a water resistant sunscreen if swimming or sweating. Immediately after towel drying. At least every 2 hours. Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10am to 2pm, wear long-sleeved shirts, pants, hats and sunglasses.
- Other Information
-
Inactive Ingredients
water, caprylic/capric triglyceride, glycerin, cyclopentasiloxane, cetyl PEG/PPG-10/1 dimethicone, thermus thermophillus ferment, polyacrylate-13, cetearyl ethylhexanoate, phenoxyethanol, polyisobutene, polyglyceryl-6 polyricinoleate, lecithin, isostearic acid, polyglyceryl-3 polyricinoleate, polyhydroxystearic acid, soidum chloride, cetearyl dimethicone, marrubium vulgare extract, helianthus annus (sunflower) see oil, disteardimonium hectorite, polysorbate 20, citrus aurantum dulcis (orange) peel oil, limonene, sorbitan isostearate, ethylhexylglycerin, panthenol, citrus nobils (mandarin orange) peel oil, disodium EDTA, sodium hyaluronate, melanin, erythritol, citric acid, vanilla planifolia fruit extract, glycose, potassium sorbate, hydrolyzed pea protein, tocopherol, PEG-8/SMDI copolymer, tetrahexyldecyl ascoorbate, tocopheryl acetate, barosma betuuna (buchu) leaf oil, soidum benzoate, homarine HCL, pentaerythrityl tetra-di-t-butylhydroxyhydrocinnamate, sodium succinate, mallis domestica fruit cell culture extract, arabidopsis thaliana (thale cress) extract, micrococcus lysate, plankton extract, ergothioneine
- Questions
- labeling
-
INGREDIENTS AND APPEARANCE
IMAGE SKINCARE PREVENTION PLUS DAILY ULTIMATE PROTECTION
homosalate, octisalate, octocrylene, zinc oxide sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60232-1022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 g in 1000 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 g in 1000 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 60 g in 1000 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 90 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONIC ACID (UNII: S270N0TRQY) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ERGOTHIONEINE (UNII: BDZ3DQM98W) ERYTHRITOL (UNII: RA96B954X6) HOMARINE HYDROCHLORIDE (UNII: 8866LNG61N) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) PEG-8/SMDI COPOLYMER (UNII: CCX72L6NY6) MALUS DOMESTICA WHOLE (UNII: 04W636S1V3) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MICROCOCCUS LUTEUS (UNII: LV6L29Z6AX) ARABIDOPSIS THALIANA (UNII: AI3L60HQ81) MARRUBIUM VULGARE (UNII: 7A72MUN24Z) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) THERMUS THERMOPHILUS (UNII: 415H64SACF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYGLYCERYL-2 ISOSTEARATE (UNII: 7B8OE71MQC) .DELTA.-TOCOPHEROL (UNII: JU84X1II0N) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ISOSTEARIC ACID (UNII: X33R8U0062) SUNFLOWER OIL (UNII: 3W1JG795YI) ORANGE OIL (UNII: AKN3KSD11B) VANILLA BEAN (UNII: Q74T35078H) AGATHOSMA BETULINA LEAF OIL (UNII: KOS935A04V) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) CETEARYL ETHYLHEXANOATE (UNII: 9M64UO4C25) LIMONENE, (+/-)- (UNII: 9MC3I34447) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60232-1022-0 7 g in 1 TUBE; Type 0: Not a Combination Product 01/31/2020 2 NDC:60232-1022-3 91 g in 1 TUBE; Type 0: Not a Combination Product 01/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/31/2020 Labeler - Swiss-American CDMO, LLC (080170933) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(60232-1022)