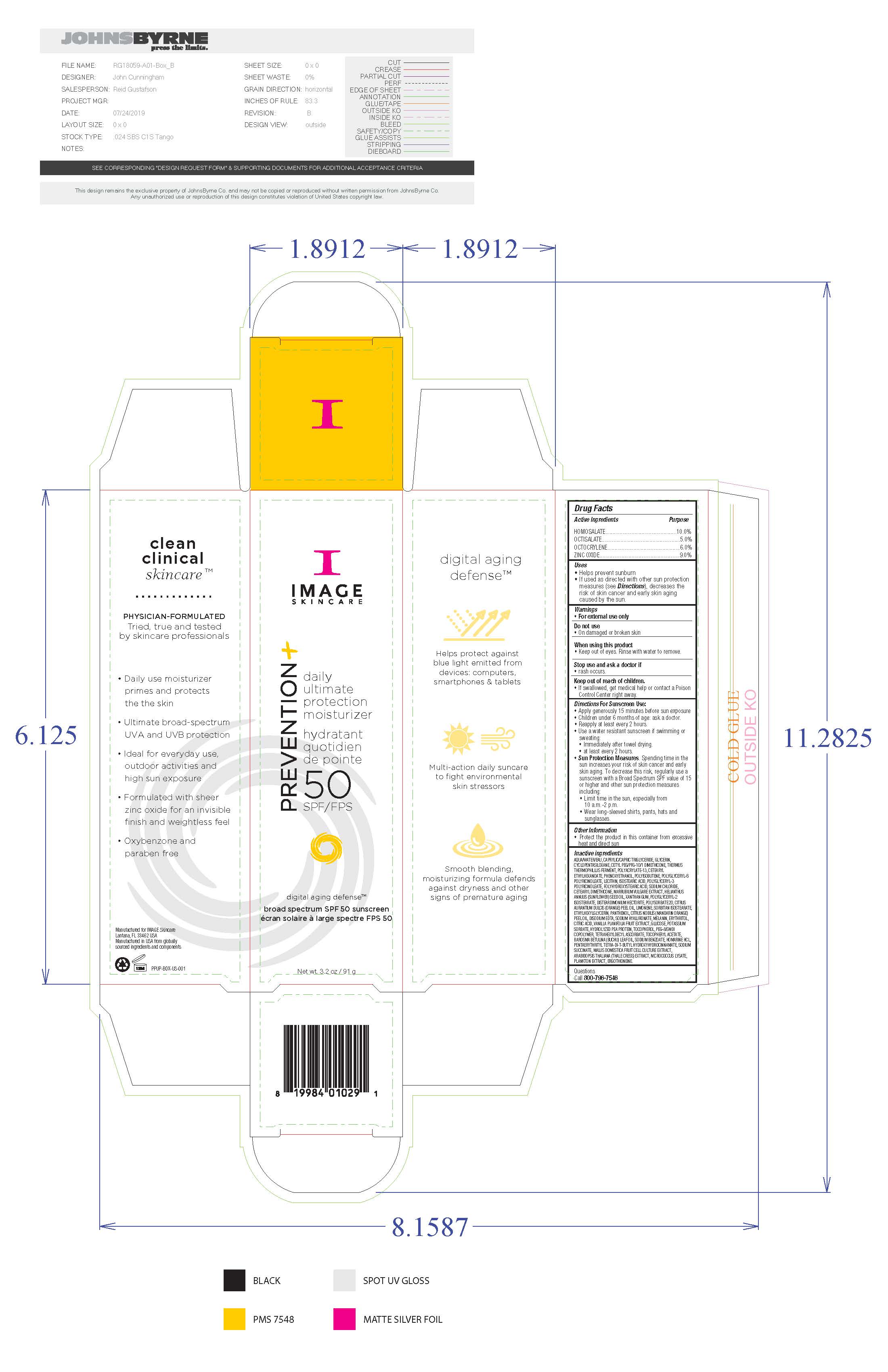
Warnings
For external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs. If swallowed, get medical help or contact a Poison Control Center right away.
Active Ingredients
Homosalate 10.0% Sunscreen
Octisalate 5.0% Sunscreen
Octocrylene 6.0% Sunscreen
Zinc Oxide 9.0% Sunscreen
Uses
Helps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Uses
Helps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions for Sunscreen Use
Apply generously 15 minutes before sun exposure. Children under 6 months of age: ask a doctor. Reapply at least every 2 hours. Use a water resistant sunscreen if swimming or sweating. Immediately after towel drying. At least every 2 hours. Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10am to 2pm, wear long-sleeved shirts, pants, hats and sunglasses.
Inactive Ingredients
water, caprylic/capric triglyceride, glycerin, cyclopentasiloxane, cetyl PEG/PPG-10/1 dimethicone, thermus thermophillus ferment, polyacrylate-13, cetearyl ethylhexanoate, phenoxyethanol, polyisobutene, polyglyceryl-6 polyricinoleate, lecithin, isostearic acid, polyglyceryl-3 polyricinoleate, polyhydroxystearic acid, soidum chloride, cetearyl dimethicone, marrubium vulgare extract, helianthus annus (sunflower) see oil, disteardimonium hectorite, polysorbate 20, citrus aurantum dulcis (orange) peel oil, limonene, sorbitan isostearate, ethylhexylglycerin, panthenol, citrus nobils (mandarin orange) peel oil, disodium EDTA, sodium hyaluronate, melanin, erythritol, citric acid, vanilla planifolia fruit extract, glycose, potassium sorbate, hydrolyzed pea protein, tocopherol, PEG-8/SMDI copolymer, tetrahexyldecyl ascoorbate, tocopheryl acetate, barosma betuuna (buchu) leaf oil, soidum benzoate, homarine HCL, pentaerythrityl tetra-di-t-butylhydroxyhydrocinnamate, sodium succinate, mallis domestica fruit cell culture extract, arabidopsis thaliana (thale cress) extract, micrococcus lysate, plankton extract, ergothioneine