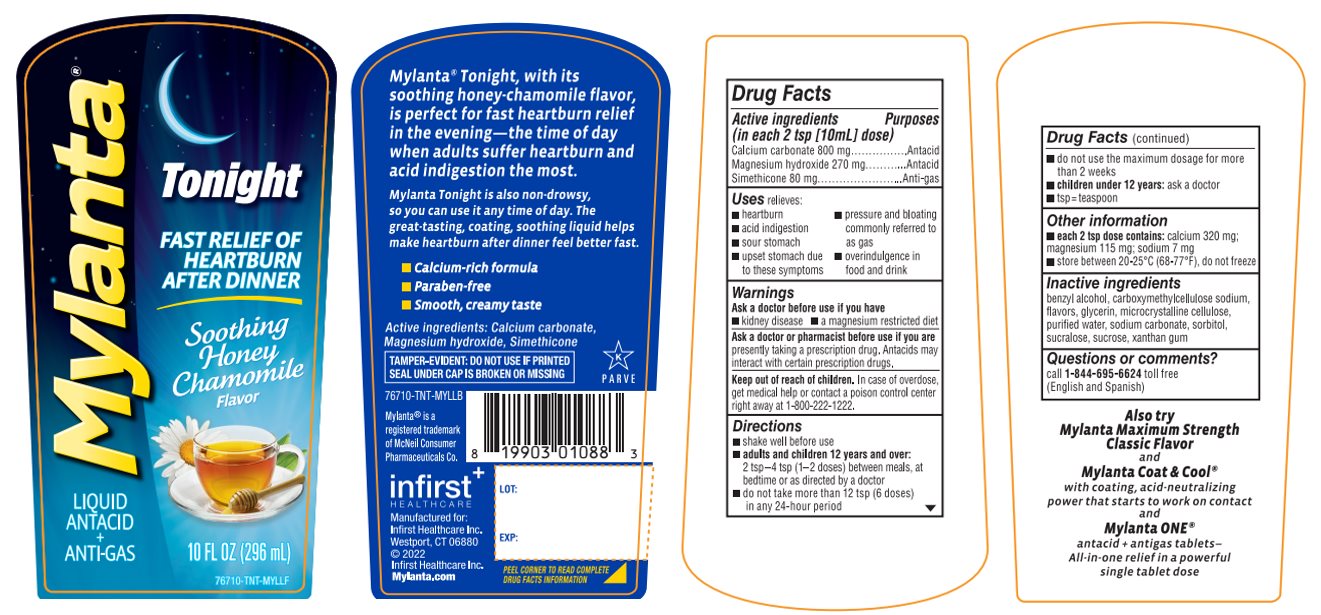
Label: MYLANTA NEW TONIGHT SOOTHING HONEY CHAMOMILE- calcium carbonate,magnesium hydroxide and simethicone suspension
- NDC Code(s): 62372-510-10
- Packager: Infirst Healthcare Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 2 tsp [10ml] dose)
- Purpose
- Uses
- Warnings
- Ask a doctor or pharmacist before use if you are
- Keep out of reach of children.
-
Directions
- •
- shake well before use
- •
-
adults and children 12 years and over:
2 tsp – 4 tsp (1-2 doses) between meals, at bedtime or as directed by a doctor - •
- do not take more than 12 tsp (6 doses) in any 24- hour period
- •
- do not use the maximum dosage for more than 2 weeks
- •
- children under 12 years: ask a doctor
- •
- tsp = teaspoon
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
MYLANTA®
LIQUID
ANTACID
+
ANTI-GASTonight
FAST RELIEF OF HEARTBURN AFTER DINNER
Soothing Honey Chamomile Flavor
10 FL OZ (296 mL)
76710-TNT-MYLLF
Mylanta® Tonight, with its soothing honey-chamomile flavor, is perfect for fast heartburn relief in the evening-the time of day when adults suffer heartburn and acid indigestion the most.
Mylanta® Tonight is also non–drowsy, so you can use it any time of the day. The great-tasting, coating, soothing liquid helps make heartburn after-dinner feel better fast.
- •
- Calcium-rich formula
- •
- Paraben-free
- •
- Smooth, creamy taste
Active ingredients: Calcium carbonate, Magnesium hydroxide, Simethicone
TAMPER-EVIDENT: DO NOT USE IF PRINTED SEAL UNDER CAP IS BROKEN OR MISSING
76710-TNT-MYLLB
Mylanta® is a registered trademark of McNeil Consumer Pharmaceuticals Co.
infirst+
HEALTHCAREManufactured for:
Infirst Healthcare Inc.
Westport, CT 06880
©2022
Infirst Healthcare Inc.
Mylanta.com -
INGREDIENTS AND APPEARANCE
MYLANTA NEW TONIGHT SOOTHING HONEY CHAMOMILE
calcium carbonate,magnesium hydroxide and simethicone suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62372-510 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D, CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 800 mg in 10 mL MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 270 mg in 10 mL DIMETHICONE (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) DIMETHICONE 80 mg in 10 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) SODIUM CARBONATE (UNII: 45P3261C7T) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE (OFF WHITE) Score Shape Size Flavor HONEY (CHAMOMILE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62372-510-10 295 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 03/21/2016 Labeler - Infirst Healthcare Inc. (079159739)