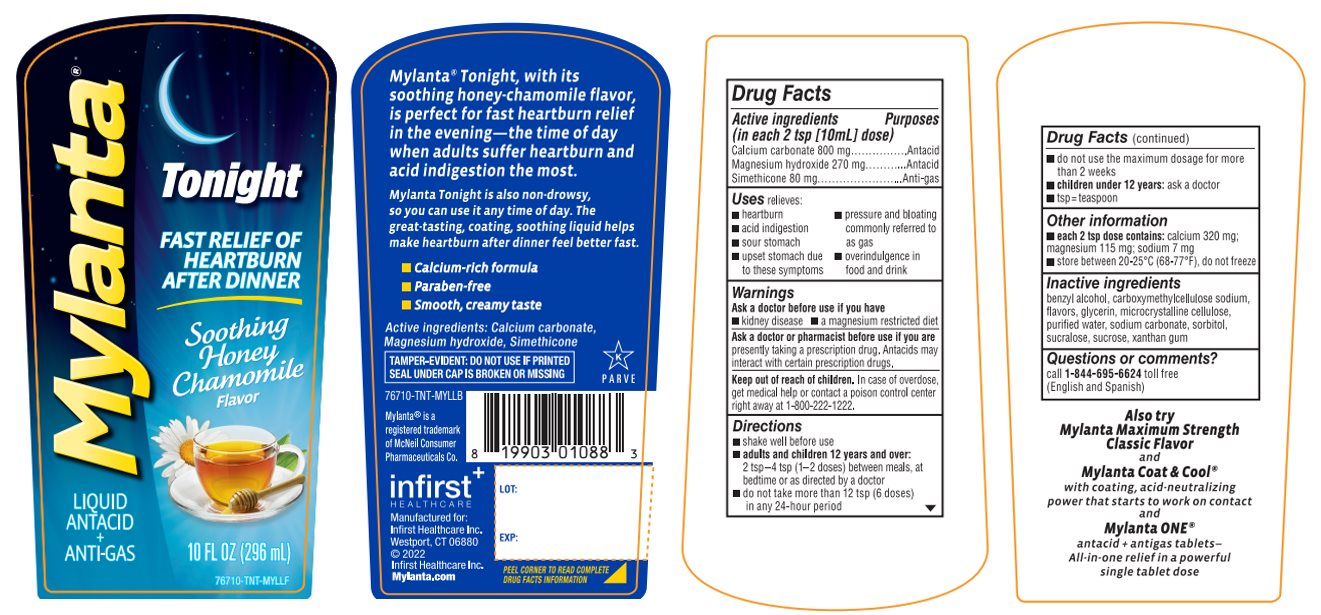
Active ingredients (in each 2 tsp [10ml] dose)
Calcium carbonate 800 mg
Magnesium hydroxide 270 mg
Simethicone 80 mg
Uses
relieves:
- •
- heartburn
- •
- pressure and bloating commonly referred to as gas
- •
- acid indigestion
- •
- sour stomach
- •
- upset stomach due to these symptoms
- •
- overindulgence in food and drink
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug. Antacids may interact with certain prescription drugs.
Keep out of reach of children.
In case of overdose, get medical help or contact a poison control center right away at 1-800-222-1222.
Directions
- •
- shake well before use
- •
-
adults and children 12 years and over:
2 tsp – 4 tsp (1-2 doses) between meals, at bedtime or as directed by a doctor - •
- do not take more than 12 tsp (6 doses) in any 24- hour period
- •
- do not use the maximum dosage for more than 2 weeks
- •
- children under 12 years: ask a doctor
- •
- tsp = teaspoon
Other information
- •
- each 2 tsp dose contains: calcium 320 mg; magnesium 115 mg; sodium 7 mg
- •
- store between 20-25°C (68-77°F), do not freeze
Inactive ingredients
benzyl alcohol, carboxymethylcellulose sodium, flavors, glycerin, microcrystalline cellulose, purified water, sodium carbonate, sorbitol, sucralose, sucrose, xanthan gum
Principal Display Panel
MYLANTA®
LIQUID
ANTACID
+
ANTI-GAS
Tonight
FAST RELIEF OF HEARTBURN AFTER DINNER
Soothing Honey Chamomile Flavor
10 FL OZ (296 mL)
76710-TNT-MYLLF
Mylanta® Tonight, with its soothing honey-chamomile flavor, is perfect for fast heartburn relief in the evening-the time of day when adults suffer heartburn and acid indigestion the most.
Mylanta® Tonight is also non–drowsy, so you can use it any time of the day. The great-tasting, coating, soothing liquid helps make heartburn after-dinner feel better fast.
- •
- Calcium-rich formula
- •
- Paraben-free
- •
- Smooth, creamy taste
Active ingredients: Calcium carbonate, Magnesium hydroxide, Simethicone
|
TAMPER-EVIDENT: DO NOT USE IF PRINTED SEAL UNDER CAP IS BROKEN OR MISSING |
76710-TNT-MYLLB
Mylanta® is a registered trademark of McNeil Consumer Pharmaceuticals Co.
infirst+
HEALTHCARE
Manufactured for:
Infirst Healthcare Inc.
Westport, CT 06880
©2022
Infirst Healthcare Inc.
Mylanta.com