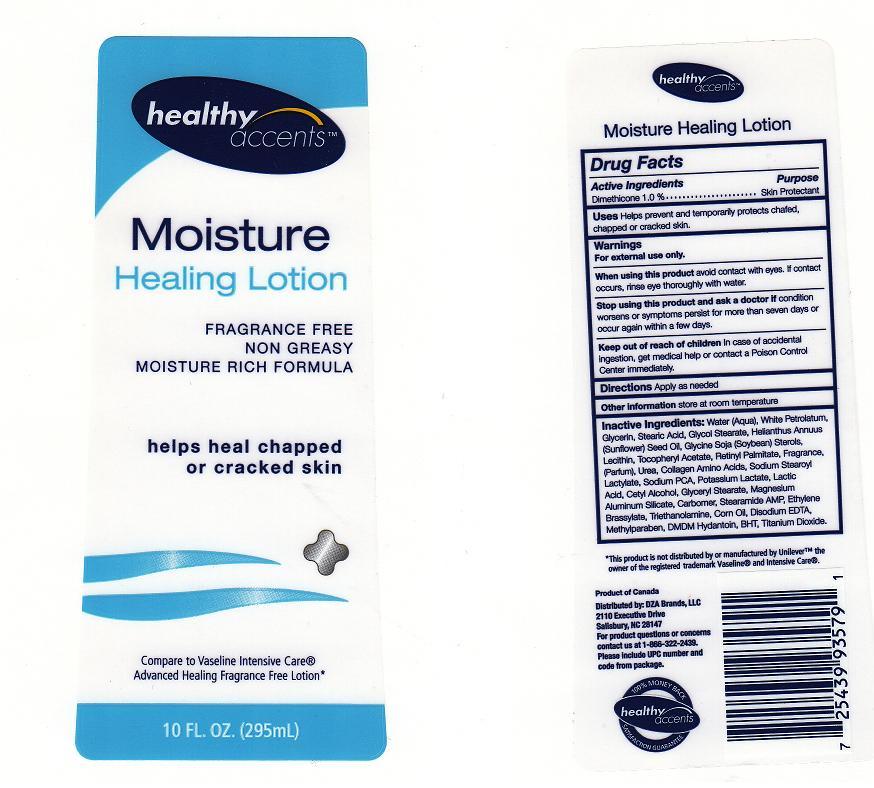
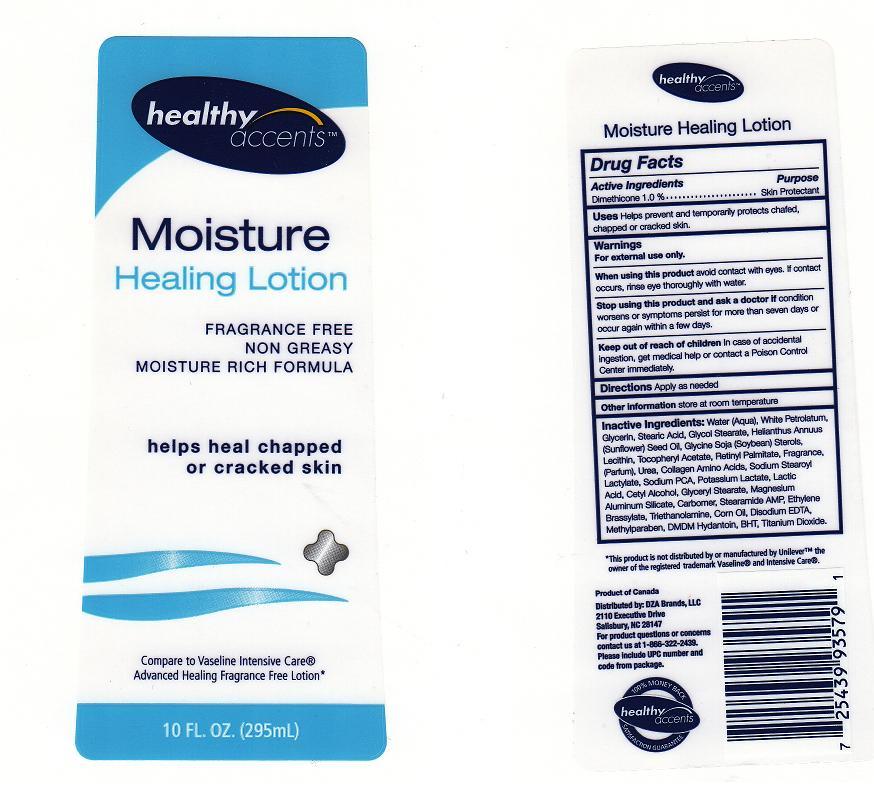
Label: HEALTHY ACCENTS MOISTURE HEALING- dimethicone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 55316-315-10 - Packager: DZA Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 25, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WARNING
- Purpose
- Uses
- Directions
-
Inactive Ingredients
Water, White Petrolatum, Glycerin, Stearic Acid, Glycol Stearate, Helianthus Annuus (Sunflower) Seed Oil, Glycine Soja (Soybean) Sterols, Lecithin, Tocopheryl Acetate, Retinyl Palmitate, Fragrance, Urea, Collagen Amino Acids, Sodium Stearoyl Lactylate, Sodium PCA, Potassium Lactate, Lactic Acid, Cetyl Alcohol, Glyceryl Stearate, Magnesium Aluminum Silicate, Carbomer, Stearamide AMP, Ethylene Brassylate, Triethanolamine, Corn Oil, Disodium EDTA, Methylparaben, DMDM Hydantoin, BHT, Titanium Dioxide
- Package Front and Back Labels
-
INGREDIENTS AND APPEARANCE
HEALTHY ACCENTS MOISTURE HEALING
dimethicone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55316-315 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 1 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCOL STEARATE (UNII: 0324G66D0E) SUNFLOWER OIL (UNII: 3W1JG795YI) SOY STEROL (UNII: PL360EPO9J) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) UREA (UNII: 8W8T17847W) SODIUM ISOSTEAROYL LACTYLATE (UNII: 8730J0D3EV) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM LACTATE (UNII: 87V1KMK4QV) LACTIC ACID (UNII: 33X04XA5AT) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) CARBOMER 934 (UNII: Z135WT9208) STEARAMIDOETHYL DIETHYLAMINE (UNII: 25FFJ4209Z) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TROLAMINE (UNII: 9O3K93S3TK) CORN OIL (UNII: 8470G57WFM) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYLPARABEN (UNII: A2I8C7HI9T) DMDM HYDANTOIN (UNII: BYR0546TOW) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55316-315-10 295 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 06/25/2010 Labeler - DZA Brands LLC (090322194)