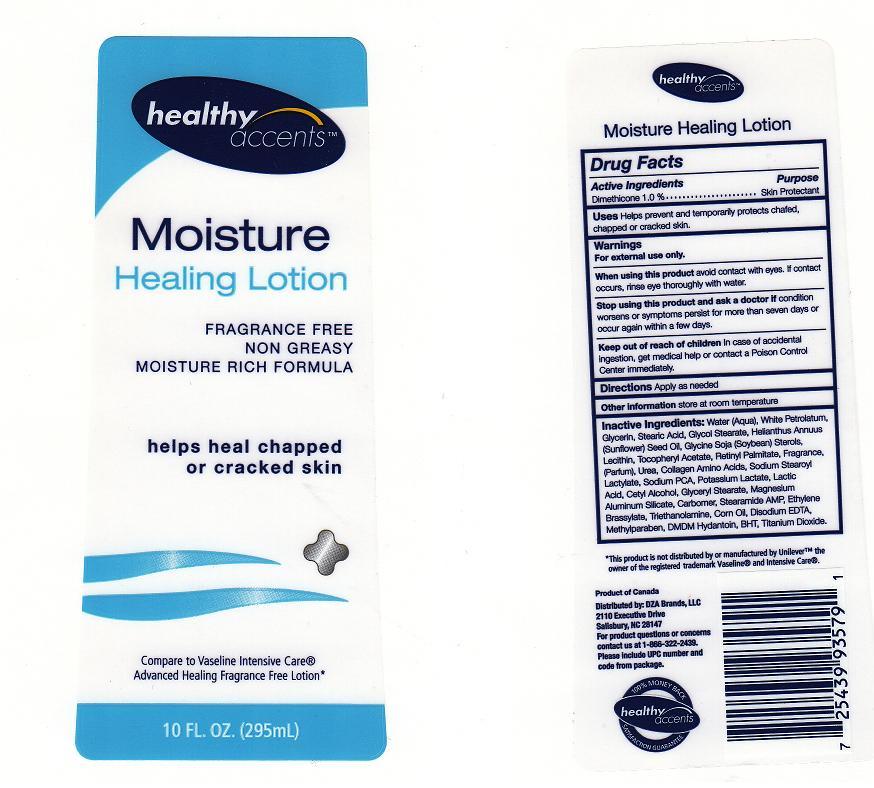
HEALTHY ACCENTS MOISTURE HEALING - dimethicone lotion
DZA Brands LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
DIMETHICONE 1.0%
WARNING
FOR EXTERNAL USE ONLY.
When using this product
AVOID CONTACT WITH EYES, IF CONTACT OCCURS, RINSE EYE THROUGHLY WITH WATER.
STOP USING THIS PRODUCT AND ASK A DOCTOR IF
CONDITION WORSENS OR SYMPTOMS PERSIST FOR MORE THAN SEVEN DAYS OR OCCUR AGAIN WITHIN A FEW DAYS.
KEEP OUT OF REACH OF CHILDREN
IN CASE OF ACCIDENTAL INGESTION, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Uses
HELPS PREVENT AND TEMPORARILY PROTECTS CHAFED, CHAPPED OR CRACKED SKIN
Directions
APPLY AS NEEDED.
Inactive Ingredients
Water, White Petrolatum, Glycerin, Stearic Acid, Glycol Stearate, Helianthus Annuus (Sunflower) Seed Oil, Glycine Soja (Soybean) Sterols, Lecithin, Tocopheryl Acetate, Retinyl Palmitate, Fragrance, Urea, Collagen Amino Acids, Sodium Stearoyl Lactylate, Sodium PCA, Potassium Lactate, Lactic Acid, Cetyl Alcohol, Glyceryl Stearate, Magnesium Aluminum Silicate, Carbomer, Stearamide AMP, Ethylene Brassylate, Triethanolamine, Corn Oil, Disodium EDTA, Methylparaben, DMDM Hydantoin, BHT, Titanium Dioxide
Package Front and Back Labels
10 OZ Front and Back Labels: hac10.jpg