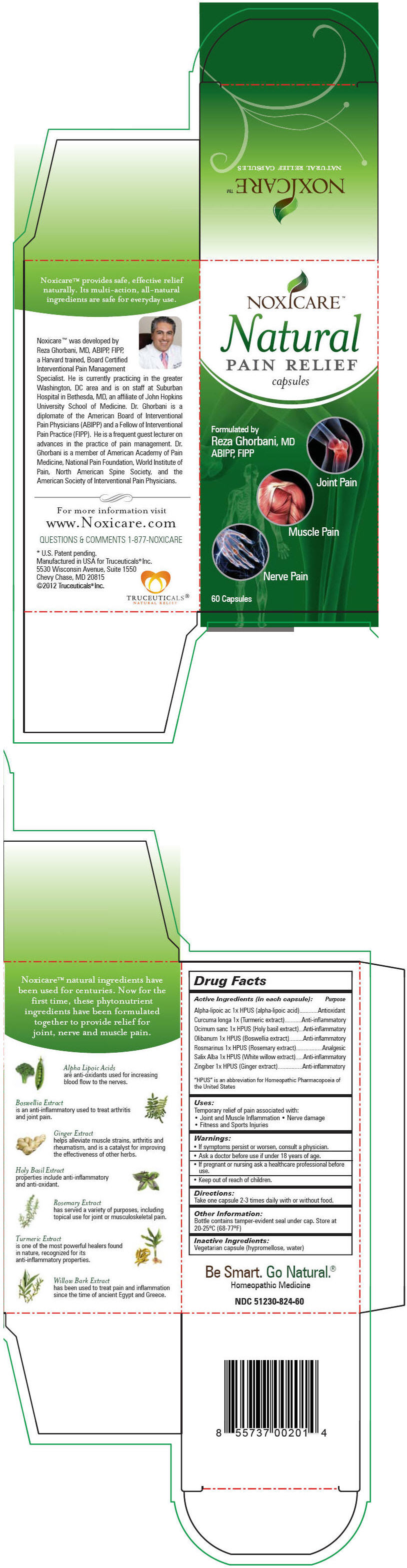
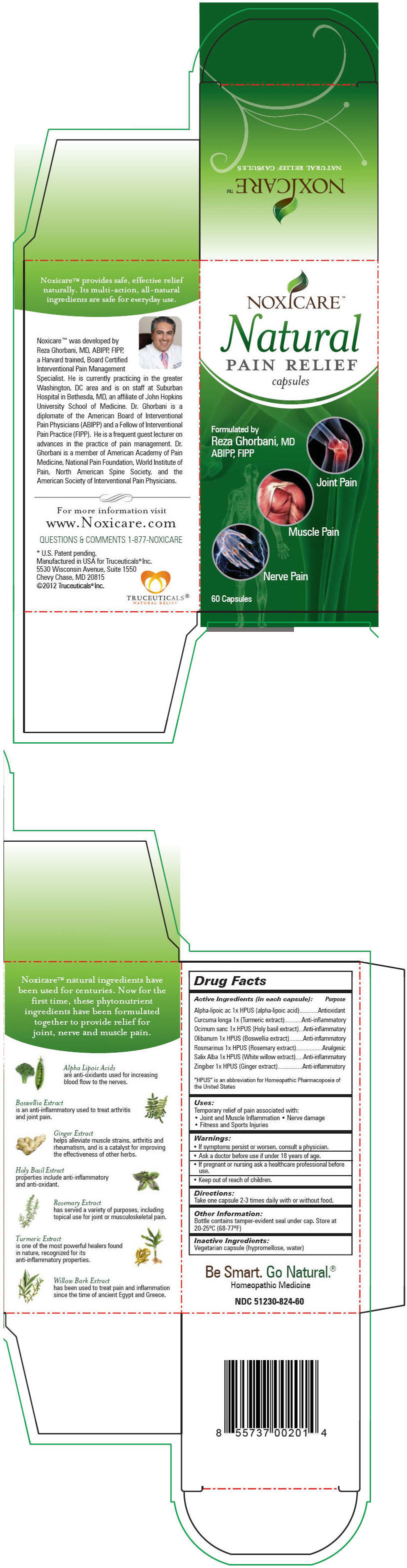
Label: NOXICARE NATURAL PAIN RELIEF- .alpha.-lipoic acid, turmeric, ocimum tenuiflorum top, indian frankincense, rosemary, salix alba bark, and ginger capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 51230-824-60 - Packager: Truceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 20, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active Ingredients (in each capsule): Purpose "HPUS" is an abbreviation for Homeopathic Pharmacopoeia of the United States Alpha-lipoic ac 1x HPUS (alpha-lipoic acid) Antioxidant Curcuma longa 1x (Turmeric extract) Anti-inflammatory Ocimum sanc 1x HPUS (Holy basil extract) Anti-inflammatory Olibanum 1x HPUS (Boswellia extract) Anti-inflammatory Rosmarinus 1x HPUS (Rosemary extract) Analgesic Salix Alba 1x HPUS (White willow extract) Anti-inflammatory Zingiber 1x HPUS (Ginger extract) Anti-inflammatory - Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- QUESTIONS & COMMENTS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 60 Capsule Bottle Carton
-
INGREDIENTS AND APPEARANCE
NOXICARE NATURAL PAIN RELIEF
.alpha.-lipoic acid, turmeric, ocimum tenuiflorum top, indian frankincense, rosemary, salix alba bark, and ginger capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51230-824 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .Alpha.-Lipoic Acid (UNII: 73Y7P0K73Y) (.Alpha.-Lipoic Acid - UNII:73Y7P0K73Y) .Alpha.-Lipoic Acid 1 [hp_X] Turmeric (UNII: 856YO1Z64F) (Turmeric - UNII:856YO1Z64F) Turmeric 1 [hp_X] Ocimum Tenuiflorum Top (UNII: 34T63W8ULS) (Ocimum Tenuiflorum Top - UNII:34T63W8ULS) Ocimum Tenuiflorum Top 1 [hp_X] Indian Frankincense (UNII: 4PW41QCO2M) (Indian Frankincense - UNII:4PW41QCO2M) Indian Frankincense 1 [hp_X] Rosemary (UNII: IJ67X351P9) (Rosemary - UNII:IJ67X351P9) Rosemary 1 [hp_X] Salix Alba Bark (UNII: 205MXS71H7) (Salix Alba Bark - UNII:205MXS71H7) Salix Alba Bark 1 [hp_X] Ginger (UNII: C5529G5JPQ) (Ginger - UNII:C5529G5JPQ) Ginger 1 [hp_X] Inactive Ingredients Ingredient Name Strength Hypromelloses (UNII: 3NXW29V3WO) Water (UNII: 059QF0KO0R) Product Characteristics Color BROWN Score no score Shape CAPSULE Size 23mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51230-824-60 1 in 1 CARTON 1 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 09/14/2012 Labeler - Truceuticals, LLC (963324954)