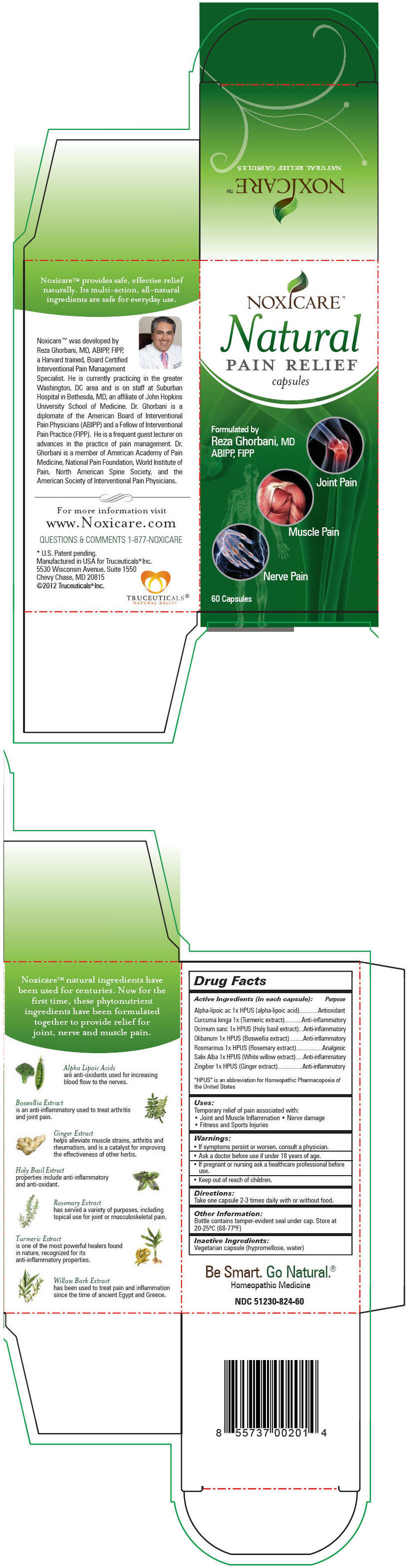
NOXICARE NATURAL PAIN RELIEF- .alpha.-lipoic acid, turmeric, ocimum tenuiflorum top, indian frankincense, rosemary, salix alba bark, and ginger capsule
Truceuticals, LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
| Active Ingredients (in each capsule): | Purpose |
| "HPUS" is an abbreviation for Homeopathic Pharmacopoeia of the United States |
| Alpha-lipoic ac 1x HPUS (alpha-lipoic acid) | Antioxidant |
| Curcuma longa 1x (Turmeric extract) | Anti-inflammatory |
| Ocimum sanc 1x HPUS (Holy basil extract) | Anti-inflammatory |
| Olibanum 1x HPUS (Boswellia extract) | Anti-inflammatory |
| Rosmarinus 1x HPUS (Rosemary extract) | Analgesic |
| Salix Alba 1x HPUS (White willow extract) | Anti-inflammatory |
| Zingiber 1x HPUS (Ginger extract) | Anti-inflammatory |
Uses
Temporary relief of pain associated with:
- Joint and Muscle Inflammation
- Nerve damage
- Fitness and Sports Injuries
Warnings
- If symptoms persist or worsen, consult a physician.
- Ask a doctor before use if under 18 years of age.
- If pregnant or nursing ask a healthcare professional before use.
- Keep out of reach of children.
Directions
Adults: Take one capsule 2-3 times daily with or without food. Children: Ask a doctor before use if under 18 years of age.
Other Information
Bottle contains tamper-evident seal under cap. Store at 20-25°C (68-77°F)
Inactive Ingredients
Vegetarian capsule (hypromellose, water)
QUESTIONS & COMMENTS
877-NOXICARE
Manufactured in USA for Truceuticals® Inc.
5530 Wisconsin Avenue, Suite 1550
Chevy Chase, MD 20815
PRINCIPAL DISPLAY PANEL - 60 Capsule Bottle Carton
NOXICARE™
Natural
PAIN RELIEF
capsules
Formulated by
Reza Ghorbani, MD
ABIPP, FIPP
Joint Pain
Muscle Pain
Nerve Pain
60 Capsules