Label: TRIBENZOR- olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coated
-
NDC Code(s):
0713-0874-30,
0713-0875-30,
0713-0876-30,
0713-0877-30, view more0713-0878-30
- Packager: Cosette Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 12, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRIBENZOR safely and effectively. See full prescribing information for TRIBENZOR.
TRIBENZOR (olmesartan medoxomil, amlodipine, hydrochlorothiazide) tablets, for oral useInitial U.S. Approval: 2010INDICATIONS AND USAGE
Tribenzor is a combination of olmesartan medoxomil, an angiotensin II receptor blocker, amlodipine, a dihydropyridine calcium channel blocker, and hydrochlorothiazide, a thiazide diuretic, indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. ( 1).
Limitations of Use
Tribenzor is not indicated for initial therapy ( 1).
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: (olmesartan medoxomil/amlodipine/hydrochlorothiazide) 20 /5 /12.5 mg, 40 /5 /12.5 mg, 40 /5 /25 mg, 40 /10 /12.5 mg, 40 /10 /25 mg ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypotension: Correct volume or salt depletion prior to administration. (
5.2).
- Monitor renal function and potassium in susceptible patients
- Increased angina or myocardial infarction with calcium channel blockers may occur upon dosage initiation or increase (
5.3).
- Observe for signs of fluid or electrolyte imbalance (
5.6).
- Exacerbation or activation of systemic lupus erythematosus (
5.8).
- Acute angle-closure glaucoma (
5.9).
- Sprue-like enteropathy has been reported. Consider discontinuation of Tribenzor in cases where no other etiology is found ( 5.10).
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2%) are dizziness, peripheral edema, headache, fatigue, nasopharyngitis, muscle spasms, nausea, upper respiratory tract infection, diarrhea, urinary tract infection, and joint swelling ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, Cosette Pharmaceuticals, Inc. at 1-800-922-1038 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
Olmesartan medoxomil ( 7.1):
- Nonsteroidal anti-inflammatory drugs (NSAIDS): May lead to increased risk of renal impairment and loss of antihypertensive effect.
- Dual inhibition of the renin-angiotensin system: Increased risk of renal impairment, hypotension, and hyperkalemia.
- Colesevelam hydrochloride: Consider administering olmesartan at least 4 hours before colesevelam hydrochloride dose.
- Lithium: Increases in serum lithium concentrations and lithium toxicity.
Amlodipine ( 7.2):
- Limit simvastatin to 20 mg daily when coadministered.
- Increased exposure to cyclosporin and tacrolimus.
- Increased amlodipine exposure when coadministered with CYP3A inhibitors Hydrochlorothiazide (
7.3):
- Antidiabetic drugs: Dosage adjustment of antidiabetic may be required.
- Cholestyramine and colestipol: Reduced absorption of thiazides.
- NSAIDs: Can reduce the diuretic, natriuretic, and antihypertensive effects of diuretics.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2022
- Hypotension: Correct volume or salt depletion prior to administration. (
5.2).
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FETAL TOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
5.2 Hypotension in Volume- or Salt-Depleted Patients
5.3 Increased Angina and/orMyocardial Infarction
5.4 Impaired Renal Function
5.5 Patients withHepatic Impairment
5.6 Electrolyte and Metabolic Imbalances
5.7 Postsympathectomy Patients
5.8 Systemic Lupus Erythematosus
5.9 Acute Myopia and Secondary Angle-Closure Glaucoma
5.10 Sprue-like Enteropathy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Drug Interactions with Olmesartan Medoxomil
7.2 Drug Interactions with Amlodipine
7.3 Drug Interactions with Hydrochlorothiazide
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 Black Patients
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Tribenzor
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE
Tribenzor is indicated for the treatment of hypertension, alone or with other antihypertensive agents, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular (CV) events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Tribenzor.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Limitations of Use
This fixed combination drug is not indicated for the initial therapy of hypertension .
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Because of the hydrochlorothiazide component, Tribenzor is contraindicated in patients with anuria, hypersensitivity to any component, or hypersensitivity to other sulfonamide-derived drugs.
Do not co-administer aliskiren with Tribenzor in patients with diabetes [See Drug Interactions (7.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Olmesartan medoxomil.Tribenzor can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Tribenzor as soon as possible [see Use in specific Populations ( 8.1].
Hydrochlorothiazide.Thiazides cross the placental barrier and appear in cord blood.
Adverse reactions include fetal or neonatal jaundice and thrombocytopenia [see Use in Specific Populations (8.1)].
5.2 Hypotension in Volume- or Salt-Depleted Patients
Olmesartan medoxomil. In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients (e.g., those being treated with high doses of diuretics) symptomatic hypotension may be anticipated after initiation of treatment with olmesartan medoxomil. Initiate treatment with Tribenzor under close medical supervision. If hypotension does occur, place the patient in the supine position and, if necessary, give an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
Amlodipine.Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. Because of the gradual onset of action, acute hypotension is unlikely.
5.3 Increased Angina and/orMyocardial Infarction
Amlodipine.Patients, particularly those with severe obstructive coronary artery disease, may develop increased frequency, duration, or severity of angina or acute myocardial infarction upon starting calcium channel blocker therapy or at the time of dosage increase. The mechanism of this effect has not been elucidated.
5.4 Impaired Renal Function
Tribenzor.
Impaired renal function was reported in 2.1% of subjects receiving Tribenzor compared to 0.2% to 1.3% of subjects receiving dual combination therapy of olmesartan medoxomil and amlodipine, olmesartan medoxomil and hydrochlorothiazide or amlodipine and hydrochlorothiazide.
If progressive renal impairment becomes evident consider withholding or discontinuing Tribenzor.
Olmesartan medoxomil.Changes in renal function occur in some individuals treated with olmesartan medoxomil as a consequence of inhibiting the renin-angiotensin-aldosterone system. In patients whose renal function may depend upon the activity of the renin-angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with ACE inhibitors and angiotensin receptor antagonists has been associated with oliguria or progressive azotemia and (rarely) with acute renal failure and/or death. Similar effects may occur in patients treated with Tribenzor due to the olmesartan medoxomil component [seeDrug Interactions (7.2) andClinical Pharmacology (12.3)].
In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen (BUN) have been reported. There has been no long-term use of olmesartan medoxomil in patients with unilateral or bilateral renal artery stenosis, but similar effects would be expected with Tribenzor because of the olmesartan medoxomil component.
Hydrochlorothiazide.Thiazides may precipitate azotemia in patients with renal disease. Cumulative effects of the drug may develop in patients with impaired renal function.
5.5 Patients withHepatic Impairment
Amlodipine.Since amlodipine is extensively metabolized by the liver and the plasma elimination half-life (t 1/2) is 56 hours in patients with severely impaired hepatic function, titrate slowly when administering to patients with severe hepatic impairment.
5.6 Electrolyte and Metabolic Imbalances
Tribenzor contains hydrochlorothiazide which can cause hypokalemia, hyponatremia and hypomagnesemia. Hypomagnesemia can result in hypokalemia which may be difficult to treat despite potassium repletion. Tribenzor also contains olmesartan, a drug that affects the RAS. Drugs that inhibit the RAS can also cause hyperkalemia.
Hydrochlorothiazide may alter glucose tolerance and raise serum levels of cholesterol and triglycerides.
Hyperuricemia may occur or frank gout may be precipitated in patients receiving thiazide therapy.
Hydrochlorothiazide decreases urinary calcium excretion and may cause elevations of serum calcium. Monitor calcium levels.
5.7 Postsympathectomy Patients
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
5.8 Systemic Lupus Erythematosus
Hydrochlorothiazide.Thiazide diuretics have been reported to cause exacerbation or activation of systemic lupus erythematosus.
5.9 Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
5.10 Sprue-like Enteropathy
Olmesartan medoxomil. Severe, chronic diarrhea with substantial weight loss has been reported in patients taking olmesartan months to years after drug initiation. Intestinal biopsies of patients often demonstrated villous atrophy. If a patient develops these symptoms during treatment with olmesartan, exclude other etiologies. Consider discontinuation of Tribenzor in cases where no other etiology is identified.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Tribenzor
In the controlled trial of Tribenzor, patients were randomized to Tribenzor (olmesartan medoxomil/amlodipine/hydrochlorothiazide 40/10/25 mg), olmesartan medoxomil/amlodipine 40/10 mg, olmesartan medoxomil/hydrochlorothiazide 40/25 mg, or amlodipine/hydrochlorothiazide 10/25 mg. Subjects who received triple combination therapy were treated between two and four weeks with one of the three dual combination therapies. Safety data from this study were obtained in 574 patients with hypertension who received Tribenzor for 8 weeks.
The frequency of adverse reactions was similar between men and women, patients <65 years of age and patients ≥65 years of age, patients with and without diabetes, and Black and non-Black patients. Discontinuations because of adverse events occurred in 4% of patients treated with Tribenzor 40/10/25 mg compared to 1% of patients treated with olmesartan medoxomil/amlodipine 40/10 mg, 2% of patients treated with olmesartan medoxomil/hydrochlorothiazide 40/25 mg, and 2% of patients treated with amlodipine/hydrochlorothiazide 10/25 mg. The most common reason for discontinuation with Tribenzor was dizziness (1%).
Dizziness was one of the most frequently reported adverse reactions with incidence of 1.4% to 3.6% in subjects continuing on dual combination therapy compared to 5.8% to 8.9% in subjects who switched to Tribenzor.
The other most frequent adverse reactions that occurred in at least 2% of subjects are presented in the table below:
Table 1 Adverse Reaction OM40/
AML10/
HCTZ25 mg
(N = 574)
n (%)OM40/
AML10 mg
(N = 596)
n (%)OM40/
HCTZ25mg
(N = 580)
n (%)AML10/
HCTZ25 mg
(N = 552)
n (%)Edema peripheral 44 (7.7) 42 (7.0) 6 (1.0) 46 (8.3) Headache 37 (6.4) 42 (7.0) 38 (6.6) 33 (6.0) Fatigue 24 (4.2) 34 (5.7) 31 (5.3) 36 (6.5) Nasopharyngitis 20 (3.5) 11 (1.8) 20 (3.4) 16 (2.9) Muscle spasms 18 (3.1) 12 (2.0) 14 (2.4) 13 (2.4) Nausea 17 (3.0) 12 (2.0) 22 (3.8) 12 (2.2) Upper respiratory tract infection 16 (2.8) 26 (4.4) 18 (3.1) 14 (2.5) Diarrhea 15 (2.6) 14 (2.3) 12 (2.1) 9 (1.6) Urinary tract infection 14 (2.4) 8 (1.3) 6 (1.0) 7 (1.3) Joint swelling 12 (2.1) 17 (2.9) 2 (0.3) 16 (2.9) Syncope was reported by 1% of Tribenzor subjects compared to 0.5% or less for the other treatment groups.
Olmesartan medoxomil
Olmesartan medoxomil has been evaluated for safety in more than 3825 patients/subjects, including more than 3275 patients treated for hypertension in controlled trials. This experience included about 900 patients treated for at least 6 months and more than 525 treated for at least 1 year. Treatment with olmesartan medoxomil was well tolerated, with an incidence of adverse reactions similar to that seen with placebo. Adverse reactions were generally mild, transient, and without relationship to the dose of olmesartan medoxomil.
Amlodipine
Amlodipine has been evaluated for safety in more than 11,000 patients in U.S. and foreign clinical trials.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of the individual components of Tribenzor. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Olmesartan medoxomil.The following adverse reactions have been reported in post-marketing experience:
Body as a Whole:asthenia, angioedema, anaphylactic reactions, peripheral edema
Gastrointestinal:vomiting, diarrhea, sprue-like enteropathy [see Warnings and Precautions (5.10)]
Metabolic and Nutritional Disorders:hyperkalemia
Musculoskeletal:rhabdomyolysis
Urogenital System:acute renal failure, increased blood creatinine
Skin and Appendages:alopecia, pruritus, urticaria
Data from one controlled trial and an epidemiologic study have suggested that high-dose olmesartan may increase cardiovascular (CV) risk in diabetic patients, but the overall data are not conclusive. The randomized, placebo-controlled, double-blind ROADMAP trial (Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention trial, n=4447) examined the use of olmesartan, 40 mg daily, vs. placebo in patients with type 2 diabetes mellitus, normoalbuminuria, and at least one additional risk factor for CV disease. The trial met its primary endpoint, delayed onset of microalbuminuria, but olmesartan had no beneficial effect on decline in glomerular filtration rate (GFR). There was a finding of increased CV mortality (adjudicated sudden cardiac death, fatal myocardial infarction, fatal stroke, revascularization death) in the olmesartan group compared to the placebo group (15 olmesartan vs. 3 placebo, HR 4.9, 95% confidence interval [CI], 1.4, 17), but the risk of non-fatal myocardial infarction was lower with olmesartan (HR 0.64, 95% CI 0.35, 1.18).
The epidemiologic study included patients 65 years and older with overall exposure of > 300,000 patient-years. In the sub-group of diabetic patients receiving high-dose olmesartan (40 mg/d) for > 6 months, there appeared to be an increased risk of death (HR 2.0, 95% CI 1.1, 3.8) compared to similar patients taking other angiotensin receptor blockers. In contrast, high-dose olmesartan use in non-diabetic patients appeared to be associated with a decreased risk of death (HR 0.46, 95% CI 0.24, 0.86) compared to similar patients taking other angiotensin receptor blockers. No differences were observed between the groups receiving lower doses of olmesartan compared to other angiotensin blockers or those receiving therapy for < 6 months.
Overall, these data raise a concern of a possible increased CV risk associated with the use of high-dose olmesartan in diabetic patients. There are, however, concerns with the credibility of the finding of increased CV risk, notably the observation in the large epidemiologic study for a survival benefit in non-diabetics of a magnitude similar to the adverse finding in diabetics.
Amlodipine.The following post-marketing event has been reported infrequently where a causal relationship is uncertain: gynecomastia. In post-marketing experience, jaundice and hepatic enzyme elevations (mostly consistent with cholestasis or hepatitis), in some cases severe enough to require hospitalization, have been reported in association with use of amlodipine. Postmarketing reporting has also revealed a possible association between extrapyramidal disorder and amlodipine.
Hydrochlorothiazide.
Non-melanoma Skin Cancer
Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
-
7 DRUG INTERACTIONS
7.1 Drug Interactions with Olmesartan Medoxomil
Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors):In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including olmesartan medoxomil, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving olmesartan medoxomil and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including olmesartan medoxomil may be attenuated by NSAIDs including selective COX-2 inhibitors.
Dual Blockade of the Renin-Angiotensin System (RAS):Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on Tribenzor and other agents that affect the RAS.
Do not co-administer aliskiren with Tribenzor in patients with diabetes [See Contraindications (4)]. Avoid use of aliskiren with Tribenzor in patients with renal impairment (GFR <60 ml/min).
Use with Colesevelam Hydrochloride:Concurrent administration of bile acid sequestering agent colesevelam hydrochloride reduces the systemic exposure and peak plasma concentration of olmesartan. Administration of olmesartan at least 4 hours prior to colesevelam hydrochloride decreased the drug interaction effect. Consider administering olmesartan at least 4 hours before the colesevelam hydrochloride dose [see Clinical Pharmacology (12.3)].
Lithium:Increases in serum lithium concentrations and lithium toxicity have been reported with concomitant use of olmesartan or thiazide diuretics. Monitor lithium levels in patients receiving Tribenzor and lithium.
7.2 Drug Interactions with Amlodipine
Simvastatin:Co-administration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily. [see Clinical Pharmacology (12.3)].
Immunosuppressants:Amlodipine may increase the systemic exposure of cyclosporine or tacrolimus when co-administered. Frequent monitoring of trough blood levels of cyclosporine and tacrolimus is recommended and adjust the dose when appropriate [see Clinical Pharmacology (12.3)].
CYP3A Inhibitors:Co-administration of amlodipine with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is co-administered with CYP3A inhibitors to determine the need for dose adjustment.
CYP3A Inducers:No information is available on the quantitative effects of CYP3A inducers on amlodipine. Blood pressure should be closely monitored when amlodipine is co-administered with CYP3A inducers.
7.3 Drug Interactions with Hydrochlorothiazide
When administered concurrently the following drugs may interact with thiazide diuretics:
Antidiabetic Drugs (oral agents and insulin):Dosage adjustment of the antidiabetic drug may be required.
Cholestyramine and Colestipol Resins:Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single dose of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85% and 43%, respectively.
Corticosteroids, ACTH:Intensified electrolyte depletion, particularly hypokalemia.
Non-steroidal Anti-inflammatory Drugs:In some patients the administration of a non-steroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics. Therefore, when hydrochlorothiazide tablets and non-steroidal anti-inflammatory agents are used concomitantly, the patients should be observed closely to determine if the desired effect of the diuretic is obtained.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Tribenzor can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death [see Clinical Considerations]. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents.
When pregnancy is detected, discontinue Tribenzor as soon as possible. Consider alternative antihypertensive therapy during pregnancy.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%–4% and 15%–20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions
Olmesartan medoxomil
Oligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension, and death.
Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of gestation. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
Closely observe infants with histories of in uteroexposure to olmesartan for hypotension, oliguria, and hyperkalemia. In neonates with a history of in uteroexposure to olmesartan, if oliguria or hypotension occur, utilize measures to maintain adequate blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and supporting renal function [see Use in Specific Populations (8.4)].
Hydrochlorothiazide
Thiazides can cross the placenta, and concentrations reached in the umbilical vein approach those in the maternal plasma. Hydrochlorothiazide, like other diuretics, can cause placental hypoperfusion. It accumulates in the amniotic fluid, with reported concentrations up to 19 times that in umbilical vein plasma. Use of thiazides during pregnancy is associated with a risk of fetal or neonatal jaundice or thrombocytopenia. Since they do not prevent or alter the course of preeclampsia, these drugs should not be used to treat hypertension in pregnant women. The use of HCTZ for other indications (e.g., heart disease) in pregnancy should be avoided.
Data
Animal Data
No reproductive studies have been conducted with the combination of olmesartan medoxomil, amlodipine and hydrochlorothiazide. However, these studies have been conducted for olmesartan medoxomil, amlodipine and hydrochlorothiazide alone, and olmesartan medoxomil and hydrochlorothiazide together.
Olmesartan medoxomil.
No teratogenic effects were observed when olmesartan medoxomil was administered to pregnant rats at oral doses up to 1000 mg/kg/day (240 times the maximum recommended human dose [MRHD] on a mg/m 2basis) or pregnant rabbits at oral doses up to 1 mg/kg/day (half the MRHD on a mg/m 2basis; higher doses could not be evaluated for effects on fetal development as they were lethal to the does). In rats, significant decreases in pup birth weight and weight gain were observed at doses ≥1.6 mg/kg/day, and delays in developmental milestones (delayed separation of ear auricular, eruption of lower incisors, appearance of abdominal hair, descent of testes, and separation of eyelids) and dose-dependent increases in the incidence of dilation of the renal pelvis were observed at doses ≥8 mg/kg/day. The no observed effect dose for developmental toxicity in rats is 0.3 mg/kg/day, about one-tenth the MRHD of 40 mg/day.
Olmesartan medoxomil and Hydrochlorothiazide.
No teratogenic effects were observed when 1.6:1 combinations of olmesartan medoxomil and hydrochlorothiazide were administered to pregnant mice at oral doses up to 1625 mg/kg/day (122 times the MRHD on a mg/m 2basis) or pregnant rats up to 1625 mg/kg/day (243 times the MRHD on a mg/m 2basis) or pregnant rabbits at oral doses up to 1 mg/kg/day (0.3 times the MRHD on a mg/m 2basis). In rats, however, fetal body weights at 1625 mg/kg/day (a toxic, sometimes lethal dose in the dams) were significantly lower than control. The no observed effect dose for developmental toxicity in rats is 162.5 mg/kg/day, about 24 times, on a mg/m 2basis, the MRHD of 40 mg olmesartan medoxomil/25 mg hydrochlorothiazide/day. (Calculations based on a 60 kg patient.)
Amlodipine.
No evidence of teratogenicity or other embryo/fetal toxicity was found when pregnant rats and rabbits were treated orally with amlodipine maleate at doses of up to 10 mg amlodipine/kg/day (respectively about 10 and 20 times the maximum recommended human dose of 10 mg amlodipine on a mg/m 2basis) during their respective periods of major organogenesis (calculations based on a patient weight of 60 kg). However, litter size was significantly decreased (by about 50%), and the number of intrauterine deaths was significantly increased (about 5-fold) in rats receiving amlodipine maleate at a dose equivalent to 10 mg amlodipine/kg/day for 14 days before mating and throughout mating and gestation. Amlodipine maleate has been shown to prolong both the gestational period and the duration of labor in rats at this dose.
8.2 Lactation
Risk Summary
There is limited information regarding the presence Tribenzor in human milk, the effects on the breastfed infant, or the effects on milk production. Amlodipine and hydrochlorothiazide are present in human milk. Olmesartan is present in rat milk [see Data] . Because of the potential for adverse effects on the nursing infant, advise a nursing woman that breastfeeding is not recommended during treatment with Tribenzor.
8.4 Pediatric Use
The safety and effectiveness of Tribenzor in pediatric patients have not been established.
8.5 Geriatric Use
Tribenzor.In a controlled clinical trial, 123 hypertensive patients treated with Tribenzor were ≥65 years of age and 18 patients were ≥75 years of age. No overall differences in the efficacy or safety of Tribenzor were observed in these patient populations; however, greater sensitivity of some older individuals cannot be ruled out. The recommended initial dose of amlodipine in patients ≥ 75 years of age is 2.5 mg, a dose not available with Tribenzor.
8.6 Hepatic Impairment
There are no studies of Tribenzor in patients with hepatic insufficiency, but both amlodipine and olmesartan medoxomil show moderate increases in exposure in patients with severe hepatic impairment. The recommended initial dose of amlodipine in patients with severe hepatic impairment is 2.5 mg, a dose not available with Tribenzor [see Warnings and Precautions (5.5)].
Amlodipine.Amlodipine is extensively metabolized by the liver and the plasma elimination half-life (t ½) is 56 hours in patients with severely impaired hepatic function.
Olmesartan medoxomil.Increases in AUC 0-∞and peak plasma concentration (C max) for olmesartan were observed with moderate hepatic impairment compared to those in matched controls with an increase in AUC of about 60%.
Hydrochlorothiazide. In patients with impaired hepatic function or progressive liver disease, minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
8.7 Renal Impairment
There are no studies of Tribenzor in patients with renal impairment. Avoid use in patients with severe renal impairment (creatinine clearance <30 mL/min).
Olmesartan medoxomil.Patients with renal insufficiency have elevated serum concentrations of olmesartan compared with patients with normal renal function. After repeated dosing, AUC was approximately tripled in patients with severe renal impairment (creatinine clearance <20 mL/min). No initial dosage adjustment is recommended for patients with moderate to marked renal impairment (creatinine clearance <40 mL/min). The pharmacokinetics of olmesartan in patients undergoing hemodialysis has not been studied.
Amlodipine.The pharmacokinetics of amlodipine are not significantly influenced by renal impairment.
Hydrochlorothiazide.Thiazide should be used with caution in patients with severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
-
10 OVERDOSAGE
There is no information on overdosage with Tribenzor in humans.
Olmesartan medoxomil.Limited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could be encountered if parasympathetic (vagal) stimulation occurs. If symptomatic hypotension should occur, supportive treatment should be initiated. The dialyzability of olmesartan is unknown.
Amlodipine.Single oral doses of amlodipine maleate equivalent to 40 mg amlodipine/kg and 100 mg amlodipine/kg in mice and rats, respectively, caused deaths. Single oral amlodipine maleate doses equivalent to 4 or more mg amlodipine/kg or higher in dogs (11 or more times the maximum recommended human dose on a mg/m 2basis) caused a marked peripheral vasodilation and hypotension.
Overdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. In humans, experience with intentional overdosage of amlodipine is limited.
If massive overdose should occur, active cardiac and respiratory monitoring should be instituted. Frequent blood pressure measurements are essential. Should hypotension occur, cardiovascular support including elevation of the extremities and the judicious administration of fluids should be initiated. If hypotension remains unresponsive to these conservative measures, administration of vasopressors (such as phenylephrine) should be considered with attention to circulating volume and urine output. Intravenous calcium gluconate may help to reverse the effects of calcium entry blockade. As amlodipine is highly protein bound, hemodialysis is not likely to be of benefit.
Hydrochlorothiazide.The most common signs and symptoms of overdose observed in humans are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias. The degree to which hydrochlorothiazide is removed by hemodialysis has not been established. The oral LD 50of hydrochlorothiazide is greater than 10 g/kg in both mice and rats, more than 1000-fold the highest recommended human dose.
-
11 DESCRIPTION
Tribenzor provided as a tablet for oral administration, is a fixed combination of olmesartan medoxomil (ARB), amlodipine (CCB), and hydrochlorothiazide (thiazide diuretic).
Olmesartan medoxomil, a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract.
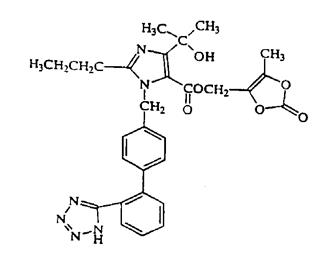
The olmesartan medoxomil component of Tribenzor is chemically described as 2,3-dihydroxy-2-butenyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[ p-(o-1 H-tetrazol-5-ylphenyl)benzyl]imidazole-5-carboxylate, cyclic 2,3-carbonate. Its empirical formula is C 29H 30N 6O 6.
The amlodipine besylate component of Tribenzor is chemically described as 3-ethyl-5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulphonate. Its empirical formula is C 20H 25ClN 2O 5•C 6H 6O 3S.
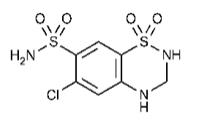
The hydrochlorothiazide component of Tribenzor is chemically described as 6-chloro-3,4-dihydro-2 H-1,2,4-benzo-thiazidiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C 7H 8ClN 3O 4S 2.
The structural formula for olmesartan medoxomil is:
The structural formula for amlodipine besylate is:
![The structural formula for amlodipine besylate is chemically described as 3 ethyl 5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzene](/dailymed/image.cfm?name=tribenzor-02.jpg&setid=2f56d0bc-236b-41a0-9fc0-21cdfea8d890)
The structural formula for hydrochlorothiazide is:
Tribenzor contains olmesartan medoxomil, a white to light yellowish-white powder or crystalline powder, amlodipine besylate, a white to off-white crystalline powder, and hydrochlorothiazide, a white or practically white, crystalline powder. The molecular weights of olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide are 558.6, 567.1, and 297.7, respectively. Olmesartan medoxomil is practically insoluble in water and sparingly soluble in methanol. Amlodipine besylate is slightly soluble in water and sparingly soluble in ethanol. Hydrochlorothiazide is slightly soluble in water but freely soluble in sodium hydroxide solution.
Each tablet of Tribenzor also contains the following inactive ingredients: silicified microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, and magnesium stearate. The color coating contains polyvinyl alcohol, macrogol/polyethylene glycol 3350, titanium dioxide, talc, iron oxide yellow (20 /5 /12.5 mg, 40 /5 /12.5 mg, 40 /5 /25 mg, 40 /10 /12.5 mg, and 40 /10 /25 mg tablets), iron oxide red (20 /5 /12.5 mg, 40 /10 /12.5 mg, and 40 /10 /25 mg tablets), and iron oxide black (20 /5 /12.5 mg tablets).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The active ingredients of Tribenzor target three separate mechanisms involved in blood pressure regulation. Specifically, amlodipine blocks the contractile effects of calcium on cardiac and vascular smooth muscle cells; olmesartan medoxomil blocks the vasoconstriction and sodium retaining effects of angiotensin II on cardiac, vascular smooth muscle, adrenal and renal cells; and hydrochlorothiazide directly promotes the excretion of sodium and chloride in the kidney leading to reductions in intravascular volume. For a more detailed description of the mechanisms of action for each individual component, see below.
Olmesartan medoxomil.Angiotensin II is formed from angiotensin I in a reaction catalyzed by ACE, kininase II. Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Olmesartan blocks the vasoconstrictor effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT 1 receptor in vascular smooth muscle. Its action is, therefore, independent of the pathways for angiotensin II synthesis.
An AT 2receptor is found also in many tissues, but this receptor is not known to be associated with cardiovascular homeostasis. Olmesartan has more than a 12,500-fold greater affinity for the AT 1receptor than for the AT 2receptor.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is a mechanism of many drugs used to treat hypertension. Angiotensin-converting enzyme inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because olmesartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and circulating angiotensin II levels do not overcome the effect of olmesartan on blood pressure.
Amlodipine. Amlodipine is a dihydropyridine calcium channel blocker that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggests that amlodipine binds to both dihydropyridine and nonhydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitrobut such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect.
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure.
Hydrochlorothiazide.Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. Indirectly, the diuretic action of hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin II, so co-administration of an angiotensin II receptor antagonist tends to reverse the potassium loss associated with these diuretics.
The mechanism of the antihypertensive effect of thiazides is not fully understood.
12.2 Pharmacodynamics
Tribenzor has been shown to be effective in lowering blood pressure. The three components of Tribenzor (olmesartan medoxomil, amlodipine, and hydrochlorothiazide) lower the blood pressure through complementary mechanisms, each working at a separate site and blocking different effects or pathways. The pharmacodynamics of each individual component is described below.
Olmesartan medoxomil.Olmesartan medoxomil doses of 2.5 to 40 mg inhibit the pressor effects of angiotensin I infusion. The duration of the inhibitory effect was related to dose, with doses of olmesartan medoxomil >40 mg giving >90% inhibition at 24 hours.
Plasma concentrations of angiotensin I and angiotensin II and plasma renin activity (PRA) increase after single and repeated administration of olmesartan medoxomil to healthy subjects and hypertensive patients. Repeated administration of up to 80 mg olmesartan medoxomil had minimal influence on aldosterone levels and no effect on serum potassium.
Amlodipine.Following administration of therapeutic doses to patients with hypertension, amlodipine produces vasodilation resulting in a reduction of supine and standing blood pressures. These decreases in blood pressure are not accompanied by a significant change in heart rate or plasma catecholamine levels with chronic dosing.
With chronic once daily oral administration, antihypertensive effectiveness is maintained for at least 24 hours. Plasma concentrations correlate with effect in both young and elderly patients. The magnitude of reduction in blood pressure with amlodipine is also correlated with the height of pretreatment elevation; thus, individuals with moderate hypertension (diastolic pressure 105-114 mmHg) had about a 50% greater response than patients with mild hypertension (diastolic pressure 90-104 mmHg). Normotensive patients experienced no clinically significant change in blood pressures (+1/-2 mmHg).
In hypertensive patients with normal renal function, therapeutic doses of amlodipine resulted in a decrease in renal vascular resistance and an increase in glomerular filtration rate and effective renal plasma flow without change in filtration fraction or proteinuria.
As with other calcium channel blockers, hemodynamic measurements of cardiac function at rest and during exercise (or pacing) in patients with normal ventricular function treated with amlodipine have generally demonstrated a small increase in cardiac index without significant influence on dP/dt or on left ventricular end diastolic pressure or volume. In hemodynamic studies, amlodipine has not been associated with a negative inotropic effect when administered in the therapeutic dose range to intact animals and man, even when co-administered with beta-blockers to man. Similar findings, however, have been observed in normal or well-compensated patients with heart failure with agents possessing significant negative inotropic effects.
Amlodipine does not change sinoatrial nodal function or atrioventricular conduction in intact animals or man. In clinical studies in which amlodipine was administered in combination with beta-blockers to patients with either hypertension or angina, no adverse effects on electrocardiographic parameters were observed.
Hydrochlorothiazide.After oral administration of hydrochlorothiazide, diuresis begins within 2 hours, peaks in about 4 hours, and lasts about 6 to 12 hours.
Drug Interactions
Alcohol, Barbiturates, or Narcotics:Potentiation of orthostatic hypotension may occur.
Skeletal muscle relaxants, non-depolarizing (e.g., tubocurarine): Possible increased responsiveness to the muscle relaxant.
12.3 Pharmacokinetics
Tribenzor. After oral administration of Tribenzor in normal healthy adults, peak plasma concentrations of olmesartan, amlodipine, and hydrochlorothiazide are reached in about 1.5 to 3 hours, 6 to 8 hours, and 1.5 to 2 hours, respectively. The rate and extent of absorption of olmesartan medoxomil, amlodipine, and hydrochlorothiazide from Tribenzor are the same as when administered as individual dosage forms. Food does not affect the bioavailability of Tribenzor.
Olmesartan medoxomil.Olmesartan medoxomil is rapidly and completely bioactivated by ester hydrolysis to olmesartan during absorption from the gastrointestinal tract. The absolute bioavailability of olmesartan medoxomil is approximately 26%. After oral administration, the C maxof olmesartan is reached after 1 to 2 hours. Food does not affect the bioavailability of olmesartan medoxomil.
Amlodipine.After oral administration of therapeutic doses of amlodipine, absorption produces peak plasma concentrations between 6 and 12 hours. Absolute bioavailability is estimated between 64% and 90%.
Hydrochlorothiazide.When plasma levels have been followed for at least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours.
Distribution
Olmesartan medoxomil.The volume of distribution of olmesartan is approximately 17 L. Olmesartan is highly bound to plasma proteins (99%) and does not penetrate red blood cells. The protein binding is constant at plasma olmesartan concentrations well above the range achieved with recommended doses.
In rats, olmesartan crossed the blood-brain barrier poorly, if at all. Olmesartan passed across the placental barrier in rats and was distributed to the fetus. Olmesartan was distributed to milk at low levels in rats.
Amlodipine.Ex vivostudies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. Steady-state plasma levels of amlodipine are reached after 7 to 8 days of consecutive daily dosing.
Hydrochlorothiazide.Hydrochlorothiazide crosses the placental but not the blood-brain barrier and is excreted in breast milk.
Metabolism and Excretion
Olmesartan medoxomil.Following the rapid and complete conversion of olmesartan medoxomil to olmesartan during absorption, there is virtually no further metabolism of olmesartan. Total plasma clearance of olmesartan is 1.3 L/h, with a renal clearance of 0.6 L/h. Approximately 35% to 50% of the absorbed dose is recovered in urine while the remainder is eliminated in feces via the bile.
Olmesartan appears to be eliminated in a biphasic manner with a terminal elimination half-life of approximately 13 hours. Olmesartan shows linear pharmacokinetics following single oral doses of up to 320 mg and multiple oral doses of up to 80 mg. Steady-state levels of olmesartan are achieved within 3 to 5 days and no accumulation in plasma occurs with once-daily dosing.
Amlodipine.Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism. Elimination from the plasma is biphasic with a terminal elimination half-life of about 30 to 50 hours. Ten percent of the parent compound and 60% of the metabolites are excreted in the urine.
Hydrochlorothiazide.Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. At least 61% of the oral dose is eliminated unchanged within 24 hours.
Specific Populations
Geriatric Patients
Olmesartan medoxomil.The pharmacokinetics of olmesartan medoxomil were studied in the elderly (≥65 years). Overall, maximum plasma concentrations of olmesartan were similar in young adults and the elderly. Modest accumulation of olmesartan was observed in the elderly with repeated dosing; AUC ѕѕ, τwas 33% higher in elderly patients, corresponding to an approximate 30% reduction in CL R.
Amlodipine.Elderly patients have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40% to 60%, and a lower initial dose may be required.
Male and Female Patients
Population pharmacokinetic analysis indicated that gender had no effect on the clearance of olmesartan and amlodipine. Female patients had approximately 20% smaller clearances of hydrochlorothiazide than male patients.
Olmesartan medoxomil.Minor differences were observed in the pharmacokinetics of olmesartan medoxomil in women compared to men. Area under the curve and C maxwere 10% to 15% higher in women than in men.
Patients with Renal Impairment
Olmesartan medoxomil.In patients with renal insufficiency, serum concentrations of olmesartan were elevated compared to subjects with normal renal function. After repeated dosing, the AUC was approximately tripled in patients with severe renal impairment (creatinine clearance <20 mL/min). The pharmacokinetics of olmesartan medoxomil in patients undergoing hemodialysis has not been studied.
Amlodipine.The pharmacokinetics of amlodipine are not significantly influenced by renal impairment.
Patients with Hepatic Impairment
Olmesartan medoxomil.Increases in AUC 0-∞and C maxwere observed in patients with moderate hepatic impairment compared to those in matched controls, with an increase in AUC of about 60%.
Amlodipine.Patients with hepatic insufficiency have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40% to 60%.
Heart Failure
Amlodipine.Patients with heart failure have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40% to 60%.
Drug Interaction Studies
Simvastatin: Co-administration of multiple doses of 10 mg of amlodipine with 80 mg simvastatin resulted in a 77% increase in exposure to simvastatin compared to simvastatin alone. [see Drug Interactions(7.2)].
CYP3A inhibitors: Co-administration of a 180 mg daily dose of diltiazem with 5 mg amlodipine in elderly hypertensive patients resulted in a 60% increase in amlodipine systemic exposure. Erythromycin co-administration in healthy volunteers did not significantly change amlodipine systemic exposure. However, strong inhibitors of CYP3A (e.g., itraconazole, clarithromycin) may increase the plasma concentrations of amlodipine to a greater extent [see Drug Interactions (7.2)].
Cyclosporine:In a prospective study in renal transplant patients, an average 40% increase in trough cyclosporine levels was observed in the presence of amlodipine. [see Drug Interactions (7.2)].
Colesevelam: Concomitant administration of 40 mg olmesartan medoxomil and 3750 mg colesevelam hydrochloride in healthy subjects resulted in 28% reduction in Cmax and 39% reduction in AUC of olmesartan. Lesser effects, 4% and 15% reduction in Cmax and AUC respectively, were observed when olmesartan medoxomil was administered 4 hours prior to colesevelam hydrochloride [see Drug Interactions (7.1)].
Cimetidine:Co-administration of amlodipine with cimetidine did not alter the pharmacokinetics of amlodipine.
Grapefruit juice:Co-administration of 240 mL of grapefruit juice with a single oral dose of amlodipine 10 mg in 20 healthy volunteers had no significant effect on the pharmacokinetics of amlodipine.
Maalox®(antacid):Co-administration of the antacid Maalox ®with a single dose of amlodipine had no significant effect on the pharmacokinetics of amlodipine.
Sildenafil:A single 100 mg dose of sildenafil in subjects with essential hypertension had no effect on the pharmacokinetic parameters of amlodipine. When amlodipine and sildenafil were used in combination, each agent independently exerted its own blood pressure lowering effect.
Atorvastatin:Co-administration of multiple 10 mg doses of amlodipine with 80 mg of atorvastatin resulted in no significant change in the steady state pharmacokinetic parameters of atorvastatin.
Digoxin:Co-administration of amlodipine with digoxin did not change serum digoxin levels or digoxin renal clearance in normal volunteers.
No significant drug interactions were reported in studies in which olmesartan medoxomil was coadministered with digoxin in healthy volunteers.
Ethanol (alcohol):Single and multiple 10 mg doses of amlodipine had no significant effect on the pharmacokinetics of ethanol.
Warfarin:Co-administration of amlodipine with warfarin did not change the warfarin prothrombin response time. No significant drug interactions were reported in studies in which olmesartan medoxomil was coadministered with warfarin in healthy volunteers.
Antacids: The bioavailability of olmesartan medoxomil was not significantly altered by the co-administration of antacids [Al(OH)3/Mg(OH)2].
-
13 NONCLINICAL TOXICOLOGY
The rationale for no or limited new toxicity from the triple combination of olmesartan medoxomil, amlodipine, and hydrochlorothiazide has already been established on the basis of the safety profile of the individual compounds or the dual combinations. To clarify the toxicological profile for Tribenzor, a 3-month repeated dose toxicity study was conducted in rats, and the results demonstrated that the combined administration of olmesartan medoxomil, amlodipine, and hydrochlorothiazide neither augment any existing toxicities of the individual agents nor induce any new toxicities and there were no toxicologically synergistic effects observed in the study.
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, mutagenicity or fertility studies have been conducted with the combination of olmesartan medoxomil, amlodipine and hydrochlorothiazide. However, these studies have been conducted for olmesartan medoxomil, amlodipine and hydrochlorothiazide alone.
Olmesartan medoxomil.Olmesartan was not carcinogenic when administered by dietary administration to rats for up to 2 years. The highest dose tested (2000 mg/kg/day) was, on a mg/m 2basis, about 480 times the MRHD of 40 mg/day. Two carcinogenicity studies conducted in mice, a 6-month gavage study in the p53 knockout mouse and a 6-month dietary administration study in the Hras2 transgenic mouse, at doses of up to 1000 mg/kg/day (on a mg/m 2basis, about 120 times the MRHD of 40 mg/day), revealed no evidence of a carcinogenic effect of olmesartan.
Both olmesartan medoxomil and olmesartan tested negative in the in vitroSyrian hamster embryo cell transformation assay and showed no evidence of genetic toxicity in the Ames (bacterial mutagenicity) test. However, both were shown to induce chromosomal aberrations in cultured cells in vitro(Chinese hamster lung) and tested positive for thymidine kinase mutations in the in vitromouse lymphoma assay. Olmesartan medoxomil tested negative in vivofor mutations in the MutaMouse intestine and kidney and for clastogenicity in mouse bone marrow (micronucleus test) at oral doses of up to 2000 mg/kg (olmesartan not tested).
Fertility of rats was unaffected by administration of olmesartan at dose levels as high as 1000 mg/kg/day (240 times the MRHD of 40 mg/day on a mg/m 2basis) in a study in which dosing was begun 2 (female) or 9 (male) weeks prior to mating. (Calculations based on a 60 kg patient.)
Amlodipine.Rats and mice treated with amlodipine maleate in the diet for up to 2 years, at concentrations calculated to provide daily dosage levels of amlodipine 0.5, 1.25, and 2.5 mg/kg/day showed no evidence of a carcinogenic effect of the drug. For the mouse, the highest dose was, on a mg/m 2basis, similar to the MRHD of amlodipine 10 mg/day. For the rat, the highest dose was, on a mg/m 2basis, about two times the MRHD (calculations based on a 60 kg patient).
Mutagenicity studies conducted with amlodipine maleate revealed no drug related effects at either the gene or chromosome level.
There was no effect on the fertility of rats treated orally with amlodipine maleate (males for 64 days and females for 14 days prior to mating) at doses of amlodipine up to 10 mg/kg/day (about 10 times the MRHD of 10 mg/day on a mg/m 2basis).
Hydrochlorothiazide.Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). These doses in mice and rats are about 117 and 39 times, respectively, the MRHD of 25 mg/day on a mg/m 2basis. (Calculations based on a 60 kg patient.) The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitroin the Ames mutagenicity assay of Salmonella typhimuriumstrains TA 98, TA 100, TA 1535, TA 1537, and TA 1538, or in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations. It was also not genotoxic in vivoin assays using mouse germinal cell chromosomes, Chinese Hamster bone marrow chromosomes, or in Drosophilasex-linked recessive lethal trait gene. Positive test results were obtained in the in vitroCHO Sister Chromatid Exchange (clastogenicity) assay, the Mouse Lymphoma Cell (mutagenicity) assay and the Aspergillus nidulansnondisjunction assay.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to mating and throughout gestation. These doses in mice and rats are about 19 and 1.5 times, respectively, the MRHD of 25 mg/day on a mg/m 2basis. (Calculations based on a 60 kg patient.)
-
14 CLINICAL STUDIES
14.1 Tribenzor
The antihypertensive efficacy of Tribenzor was studied in a double-blind, active-controlled study in hypertensive patients. A total of 2492 patients with hypertension (mean baseline blood pressure 169/101 mmHg) received olmesartan medoxomil/amlodipine/hydrochlorothiazide 40/10/25 mg (627 patients), olmesartan medoxomil/amlodipine 40/10 mg (628 patients), olmesartan medoxomil/hydrochlorothiazide 40/25 mg (637 patients), or amlodipine/hydrochlorothiazide 10/25 mg (600 patients). Each subject was randomized to one of the three dual therapy combinations for two to four weeks. Patients were then randomized to continue on the dual therapy they were receiving or to receive triple therapy. A total of 53% of patients were male, 19% were 65 years or older, 67% were white, 30% were black, and 15% were diabetic.
After 8 weeks of treatment, the triple combination therapy produced greater reductions in both systolic and diastolic blood pressures (p< 0.0001) compared to each of the 3 dual combination therapies. The full blood pressure lowering effects were attained within 2 weeks after a change in dose.
The seated blood pressure reductions attributable to the addition of a single high-dose drug to each high-dose dual drug combination are shown in Table 2.
Table 2 Additional blood pressure reductions on high-dose Tribenzor compared to high doses of dual combination drugs Start on Adding BP reduction* Olmesartan medoxomil 40 /
amlodipine 10 mgHCTZ 25 mg 8.4/4.5 mmHg Olmesartan medoxomil 40 /
HCTZ 25 mgAmlodipine 10 mg 7.6/5.4 mmHg Amlodipine 10 /
HCTZ 25 mgOlmesartan medoxomil 40 mg 8.1/5.4 mmHg *all highly statistically significant.
There were no apparent differences in terms of seated diastolic blood pressure (SeDBP) or seated systolic blood pressure (SeSBP) reductions in black and non-black patients treated with Tribenzor [see Use in Specific Populations (8.8)].
There were no apparent differences in terms of SeDBP or SeSBP reductions in diabetic and non-diabetic patients treated with Tribenzor.
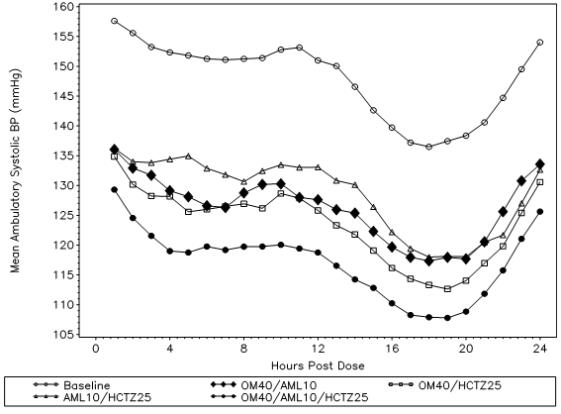
A total of 440 patients participated in the ambulatory blood pressure monitoring portion of the study. Over the 24-hour period, there was a greater reduction in diastolic and systolic ambulatory blood pressure for olmesartan medoxomil/amlodipine/hydrochlorothiazide 40/10/25 mg compared to each of the dual combination therapies (see Figure 1 and Figure 2).
Figure1:Mean Ambulatory Diastolic Blood Pressure at Endpoint by Treatment and Hour
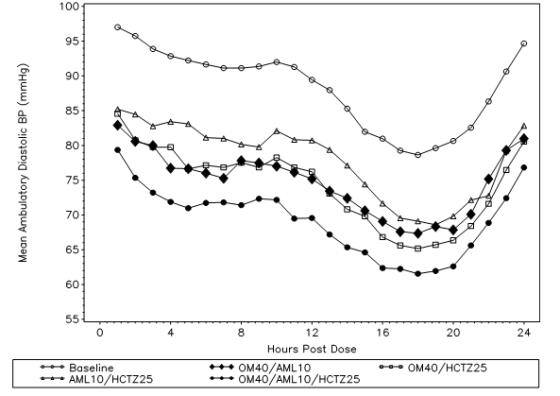
Figure2:Mean Ambulatory Systolic Blood Pressure at Endpoint by Treatment and Hour
The blood pressure lowering effects of lower dose strengths of Tribenzor (olmesartan medoxomil/amlodipine/hydrochlorothiazide 20/5/12.5 mg, 40/5/12.5 mg, 40/10/12.5 mg, and 40/5/25 mg) have not been studied.
All of the dose strengths of the triple combination are expected to provide superior blood pressure lowering effects compared to their respective mono and dual combination components. The order of the blood pressure lowering effects among the different dose strengths of Tribenzor (olmesartan medoxomil /amlodipine /hydrochlorothiazide) is expected to be 20/5/12.5 mg < 40/5/12.5 mg < (40/10/12.5 mg ≈ 40/5/25 mg) < 40/10/25 mg.
There are no trials of Tribenzor demonstrating reductions in cardiovascular risk in patients with hypertension, but at least one pharmacologically similar drug has demonstrated such benefits.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tribenzor tablets contain olmesartan medoxomil, amlodipine besylate at a dose equivalent to 5 or 10 mg amlodipine, and hydrochlorothiazide in the strengths described below.
Tribenzor tablets are differentiated by tablet color/size and are debossed with an individual product tablet code on one side. Tribenzor tablets are supplied for oral administration in the following strength and package configurations:
Tablet Strength
(OM/AML equivalent/HCTZ)Package Configuration NDC# Product Code Tablet Color 20 /5 /12.5 mg Bottle of 30
Bottle of 90
10 blisters of 100713-0874-30
Not available
Not availableC51 Orange white 40 /5 /12.5 mg Bottle of 30
Bottle of 90
10 blisters of 100713-0875-30
Not available
Not availableC53 Light yellow 40 /5 /25 mg Bottle of 30
Bottle of 90
10 blisters of 100713-0876-30
Not available
Not availableC54 Light yellow 40 /10 /12.5 mg Bottle of 30
Bottle of 90
10 blisters of 100713-0877-30
Not available
Not availableC55 Grayish red 40 /10 /25 mg Bottle of 30
Bottle of 90
10 blisters of 100713-0878-30
Not available
Not availableC57 Grayish red Store at 25ºC (77ºF); excursions permitted to 15ºC-30ºC (59ºF-86ºF) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Pregnancy: Tell female patients of childbearing age about the consequences of exposure to Tribenzor during pregnancy. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible [see Warnings and Precautions (5.1)and Use in Specific Populations (8.1)] .
Lactation: Advise nursing women not to breastfeed during treatment with Tribenzor [see Use in Specific Populations (8.2)] .
Symptomatic Hypotension:Advise patients that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to the prescribing physician. Tell patients that if syncope occurs, Tribenzor should be discontinued until the physician has been consulted. Tell patients that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope .
Non-melanoma Skin Cancer:Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening.
Potassium Supplements:Advise patients not to use potassium supplements or salt substitutes containing potassium without consulting their healthcare provider.
Acute myopia and secondary angle-closure glaucoma: Advise patients to discontinue Tribenzor and seek immediate medical attention if they experience symptoms of acute myopia or secondary angle-closure glaucoma [see Warnings and Precautions (5.9)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0713-0874 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLMESARTAN MEDOXOMIL (UNII: 6M97XTV3HD) (OLMESARTAN - UNII:8W1IQP3U10) OLMESARTAN MEDOXOMIL 20 mg AMLODIPINE BESYLATE (UNII: 864V2Q084H) (AMLODIPINE - UNII:1J444QC288) AMLODIPINE 5 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color orange Score no score Shape ROUND Size 8mm Flavor Imprint Code C51 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0713-0874-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA200175 09/01/2022 TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0713-0875 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLMESARTAN MEDOXOMIL (UNII: 6M97XTV3HD) (OLMESARTAN - UNII:8W1IQP3U10) OLMESARTAN MEDOXOMIL 40 mg AMLODIPINE BESYLATE (UNII: 864V2Q084H) (AMLODIPINE - UNII:1J444QC288) AMLODIPINE 5 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color yellow Score no score Shape ROUND Size 10mm Flavor Imprint Code C53 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0713-0875-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA200175 09/01/2022 TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0713-0876 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLMESARTAN MEDOXOMIL (UNII: 6M97XTV3HD) (OLMESARTAN - UNII:8W1IQP3U10) OLMESARTAN MEDOXOMIL 40 mg AMLODIPINE BESYLATE (UNII: 864V2Q084H) (AMLODIPINE - UNII:1J444QC288) AMLODIPINE 5 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 25 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color yellow Score no score Shape OVAL Size 15mm Flavor Imprint Code C54 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0713-0876-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA200175 09/01/2022 TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0713-0877 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLMESARTAN MEDOXOMIL (UNII: 6M97XTV3HD) (OLMESARTAN - UNII:8W1IQP3U10) OLMESARTAN MEDOXOMIL 40 mg AMLODIPINE BESYLATE (UNII: 864V2Q084H) (AMLODIPINE - UNII:1J444QC288) AMLODIPINE 10 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color red Score no score Shape ROUND Size 10mm Flavor Imprint Code C55 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0713-0877-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA200175 09/01/2022 TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0713-0878 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLMESARTAN MEDOXOMIL (UNII: 6M97XTV3HD) (OLMESARTAN - UNII:8W1IQP3U10) OLMESARTAN MEDOXOMIL 40 mg AMLODIPINE BESYLATE (UNII: 864V2Q084H) (AMLODIPINE - UNII:1J444QC288) AMLODIPINE 10 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 25 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color red Score no score Shape OVAL Size 15mm Flavor Imprint Code C57 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0713-0878-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA200175 09/01/2022 Labeler - Cosette Pharmaceuticals, Inc. (116918230)










