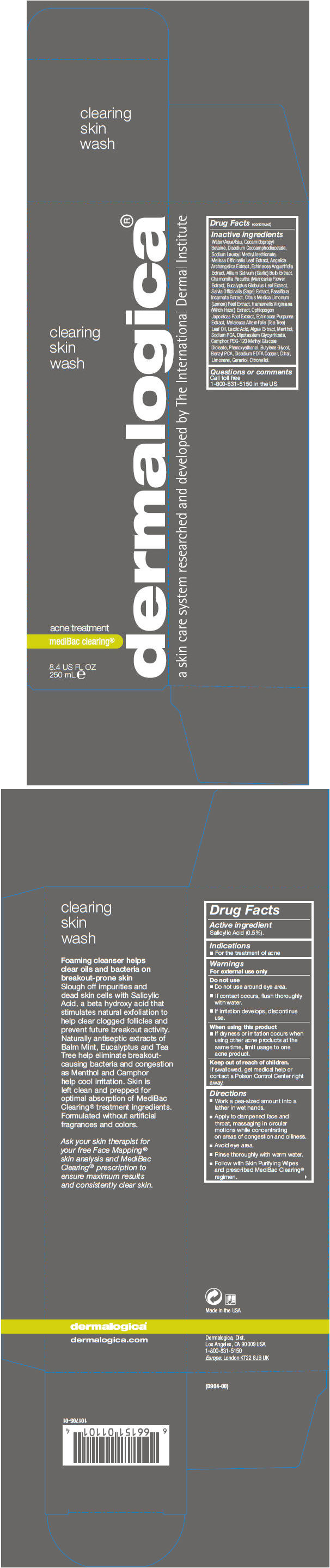
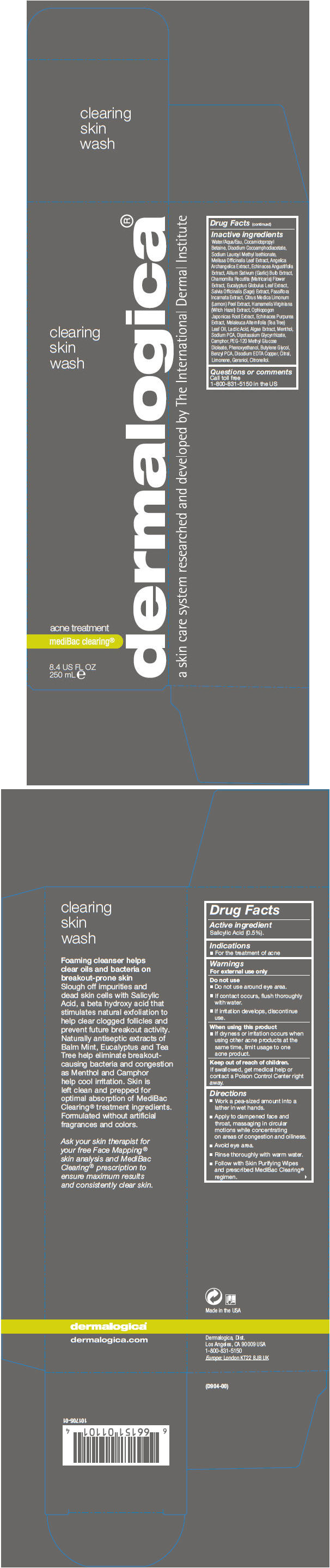
Label: CLEARING SKIN WASH- salicylic acid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 68479-040-00, 68479-040-01, 68479-040-02, 68479-040-03, view more68479-040-04 - Packager: Dermalogica, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 19, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- PURPOSE
- Warnings
- Directions
-
Inactive ingredients
Water/Aqua/Eau, Cocamidopropyl Betaine, Disodium Cocoamphodiacetate, Sodium Lauroyl Methyl Isethionate, Melissa Officinalis Leaf Extract, Angelica Archangelica Extract, Echinacea Angustifolia Extract, Allium Sativum (Garlic) Bulb Extract, Chamomilla Recutita (Matricaria) Flower Extract, Eucalyptus Globulus Leaf Extract, Salvia Officinalis (Sage) Extract, Passiflora Incarnata Extract, Citrus Medica Limonum (Lemon) Peel Extract, Hamamelis Virginiana (Witch Hazel) Extract, Ophiopogon Japonicas Root Extract, Echinacea Purpurea Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Lactic Acid, Algae Extract, Menthol, Sodium PCA, Dipotassium Glycyrrhizate, Camphor, PEG-120 Methyl Glucose Dioleate, Phenoxyethanol, Butylene Glycol, Benzyl PCA, Disodium EDTA Copper, Citral, Limonene, Geraniol, Citronellol.
- Questions or comments
- PRINCIPAL DISPLAY PANEL - 250 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
CLEARING SKIN WASH
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68479-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) Disodium Cocoamphodiacetate (UNII: 18L9G3U51M) Sodium Lauroyl Methyl Isethionate (UNII: II6VCD3S6R) Melissa Officinalis Leaf (UNII: 50D2ZE9219) Angelica Archangelica Root (UNII: DTN01M69SN) Echinacea Angustifolia (UNII: VB06AV5US8) Garlic (UNII: V1V998DC17) Chamomile (UNII: FGL3685T2X) Eucalyptus Globulus Leaf (UNII: S546YLW6E6) Salvia Officinalis Root (UNII: 236QY0A1BL) Lemon Peel (UNII: 72O054U628) Hamamelis Virginiana Top (UNII: UDA30A2JJY) Ophiopogon Japonicus Root (UNII: 90PS6JV9GZ) Echinacea Purpurea (UNII: QI7G114Y98) Tea Tree Oil (UNII: VIF565UC2G) Lactic Acid (UNII: 33X04XA5AT) Menthol, Unspecified Form (UNII: L7T10EIP3A) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Glycyrrhizinate Dipotassium (UNII: CA2Y0FE3FX) Camphor (Synthetic) (UNII: 5TJD82A1ET) PEG-120 Methyl Glucose Dioleate (UNII: YM0K64F20V) Phenoxyethanol (UNII: HIE492ZZ3T) Butylene Glycol (UNII: 3XUS85K0RA) Benzyl PCA (UNII: OUS14HJ3HZ) Disodium EDTA-Copper (UNII: 6V475AX06U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68479-040-02 1 in 1 CARTON 1 250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:68479-040-00 2 mL in 1 POUCH; Type 0: Not a Combination Product 3 NDC:68479-040-03 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC:68479-040-01 7 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 5 NDC:68479-040-04 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part358H 12/30/2009 Labeler - Dermalogica, Inc. (177698560) Establishment Name Address ID/FEI Business Operations Cosway 620899877 MANUFACTURE(68479-040) Establishment Name Address ID/FEI Business Operations Diamond Wipes 161104729 MANUFACTURE(68479-040)