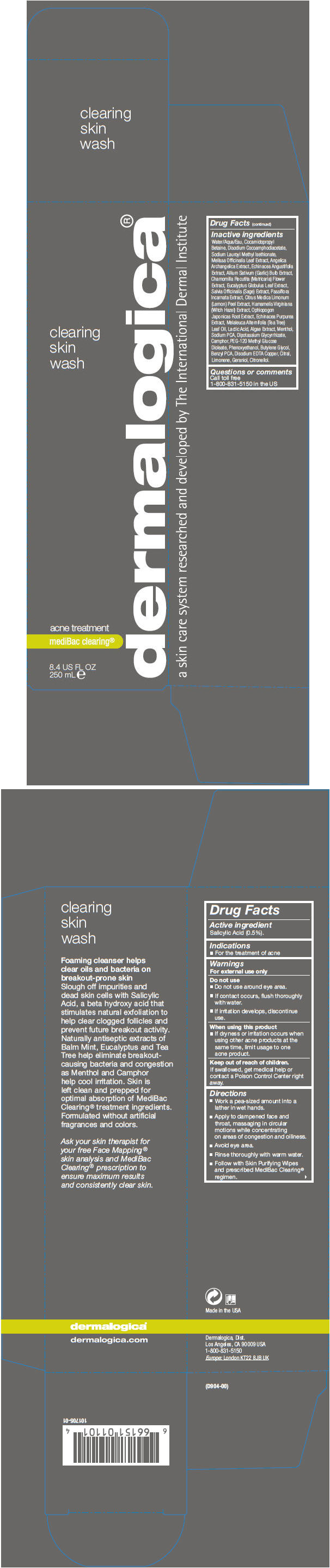
Warnings
For external use only
Do not use
- Do not use around eye area.
- If contact occurs, flush thoroughly with water.
- If irritation develops, discontinue use.
Directions
- Work a pea-sized amount into a lather in wet hands.
- Apply to dampened face and throat, massaging in circular motions while concentrating on areas of congestion and oiliness.
- Avoid eye area.
- Rinse thoroughly with warm water.
- Follow with Skin Purifying Wipes and prescribed MediBac Clearing® regimen.
Inactive ingredients
Water/Aqua/Eau, Cocamidopropyl Betaine, Disodium Cocoamphodiacetate, Sodium Lauroyl Methyl Isethionate, Melissa Officinalis Leaf Extract, Angelica Archangelica Extract, Echinacea Angustifolia Extract, Allium Sativum (Garlic) Bulb Extract, Chamomilla Recutita (Matricaria) Flower Extract, Eucalyptus Globulus Leaf Extract, Salvia Officinalis (Sage) Extract, Passiflora Incarnata Extract, Citrus Medica Limonum (Lemon) Peel Extract, Hamamelis Virginiana (Witch Hazel) Extract, Ophiopogon Japonicas Root Extract, Echinacea Purpurea Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Lactic Acid, Algae Extract, Menthol, Sodium PCA, Dipotassium Glycyrrhizate, Camphor, PEG-120 Methyl Glucose Dioleate, Phenoxyethanol, Butylene Glycol, Benzyl PCA, Disodium EDTA Copper, Citral, Limonene, Geraniol, Citronellol.