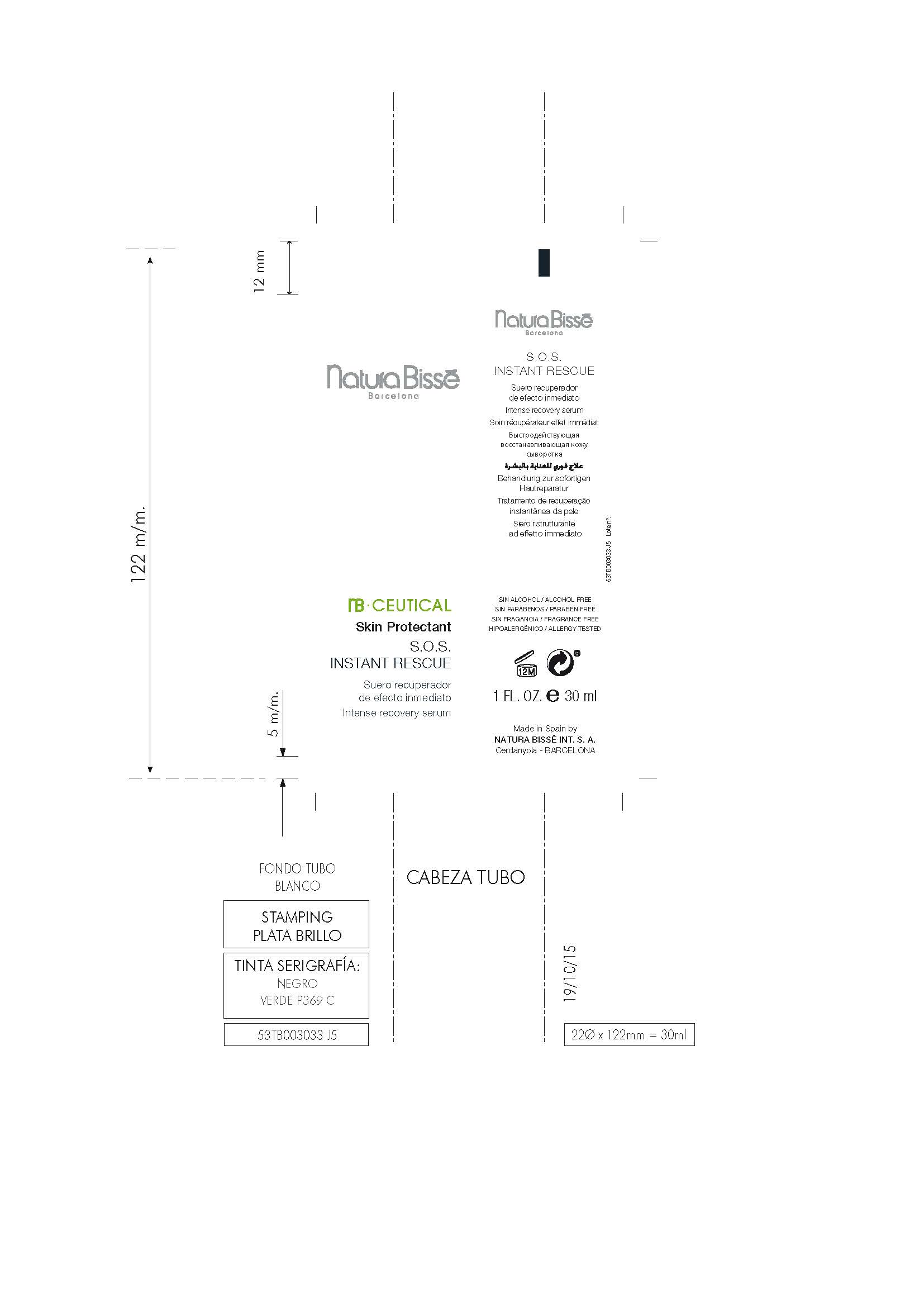
Label: S.O.S. INSTANT RESCUE- dimethicone gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 63730-391-01, 63730-391-02, 63730-391-03, 63730-391-04, view more63730-391-05, 63730-391-06 - Packager: NATURA BISSÉ INTERNATIONAL, S.A.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 14, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- INDICATIONS AND USAGE
- ACTIVE INGREDIENT
- DOSAGE AND ADMINISTRATION
-
INACTIVE INGREDIENTS
Water, Pentylene Glycol, Calendula Officinalis Flower Extract, Methyl Gluceth-10,
Propylene Glycol, Butylene Glycol, Aloe Barbadensis Leaf Extract, Sodium Acrylates
Copolymer, Panthenol, Allantoin, Lecithin, Glycerin, Arginine, Laureth-3, Arnica
Montana Flower Extract, Hydroxyethylcellulose, Propolis Extract, Disodium EDTA,
Potassium Sorbate, Acetyl Dipeptide-1 Cetyl Ester, Urea, Lactic Acid, Sodium Lactate,
Serine, Sorbitol, Pseudoalteromonas Ferment Extract, Sodium Benzoate, Hydrolyzed
Wheat Protein, Sodium Chloride, Hydrolyzed Soy Protein, Ascorbic Acid, Phenoxyethanol,
Caprylyl Glycol, Xanthan Gum, Citric Acid, Tripeptide-10 Citrulline, Carbomer,
Tripeptide-1, Triethanolamine. - WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- OTHER INFORMATION
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
S.O.S. INSTANT RESCUE
dimethicone gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63730-391 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 1 mg in 100 mg Inactive Ingredients Ingredient Name Strength BIOTINOYL TRIPEPTIDE-1 (UNII: O6380721VA) PROPOLIS WAX (UNII: 6Y8XYV2NOF) 1,3-BIS(BENZOTHIAZOL-2-YLTHIOMETHYL)UREA (UNII: 0214C2T14J) LACTIC ACID, D- (UNII: 3Q6M5SET7W) SODIUM LACTATE (UNII: TU7HW0W0QT) ALTEROMONAS MACLEODII (UNII: BPX036043D) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM BENZOATE (UNII: OJ245FE5EU) TRIPEPTIDE-10 CITRULLINE (UNII: 2732R0E76W) PROPYLENE GLYCOL, (R)- (UNII: 602HN5L69H) ALLANTOIN, (+)- (UNII: XDK458E1J9) ALOE VERA LEAF (UNII: ZY81Z83H0X) ARGININE, D- (UNII: R54Z304Z7C) LAURETH-3 (UNII: F32E4CB0UJ) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CETYL HYDROXYETHYLCELLULOSE (550000 MW) (UNII: 2MIM45ZIL3) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) N-ACETYL DIPEPTIDE-1 (UNII: HA41Z1UF8D) ASCORBIC ACID (UNII: PQ6CK8PD0R) CITRIC ACID ACETATE (UNII: DSO12WL7AU) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL, L- (UNII: 01Q0586BG1) TROLAMINE (UNII: 9O3K93S3TK) SODIUM ACRYLATE (UNII: 7C98FKB43H) PANTHENOL (UNII: WV9CM0O67Z) PENTYLENE GLYCOL (UNII: 50C1307PZG) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER 940 (UNII: 4Q93RCW27E) WATER (UNII: 059QF0KO0R) SERINE, D- (UNII: 1K77H2Z9B1) HYDROLYZED WHEAT PROTEIN (ENZYMATIC, 3000 MW) (UNII: J2S07SB0YL) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) EGTA DISODIUM (UNII: 7T6F42A6GN) METHYL GLUCETH-10 (UNII: N0MWT4C7WH) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63730-391-01 30 mg in 1 JAR; Type 0: Not a Combination Product 02/29/2016 03/14/2016 2 NDC:63730-391-02 2 mg in 1 JAR; Type 0: Not a Combination Product 02/29/2016 03/14/2016 3 NDC:63730-391-03 7 mg in 1 JAR; Type 0: Not a Combination Product 02/29/2016 03/14/2016 4 NDC:63730-391-04 30 mg in 1 TUBE; Type 0: Not a Combination Product 03/14/2016 5 NDC:63730-391-05 2 mg in 1 TUBE; Type 0: Not a Combination Product 03/14/2016 6 NDC:63730-391-06 7 mg in 1 TUBE; Type 0: Not a Combination Product 03/14/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 02/29/2016 Labeler - NATURA BISSÉ INTERNATIONAL, S.A. (464431576) Establishment Name Address ID/FEI Business Operations NATURA BISSÉ INTERNATIONAL, S.A. 464431576 manufacture(63730-391)