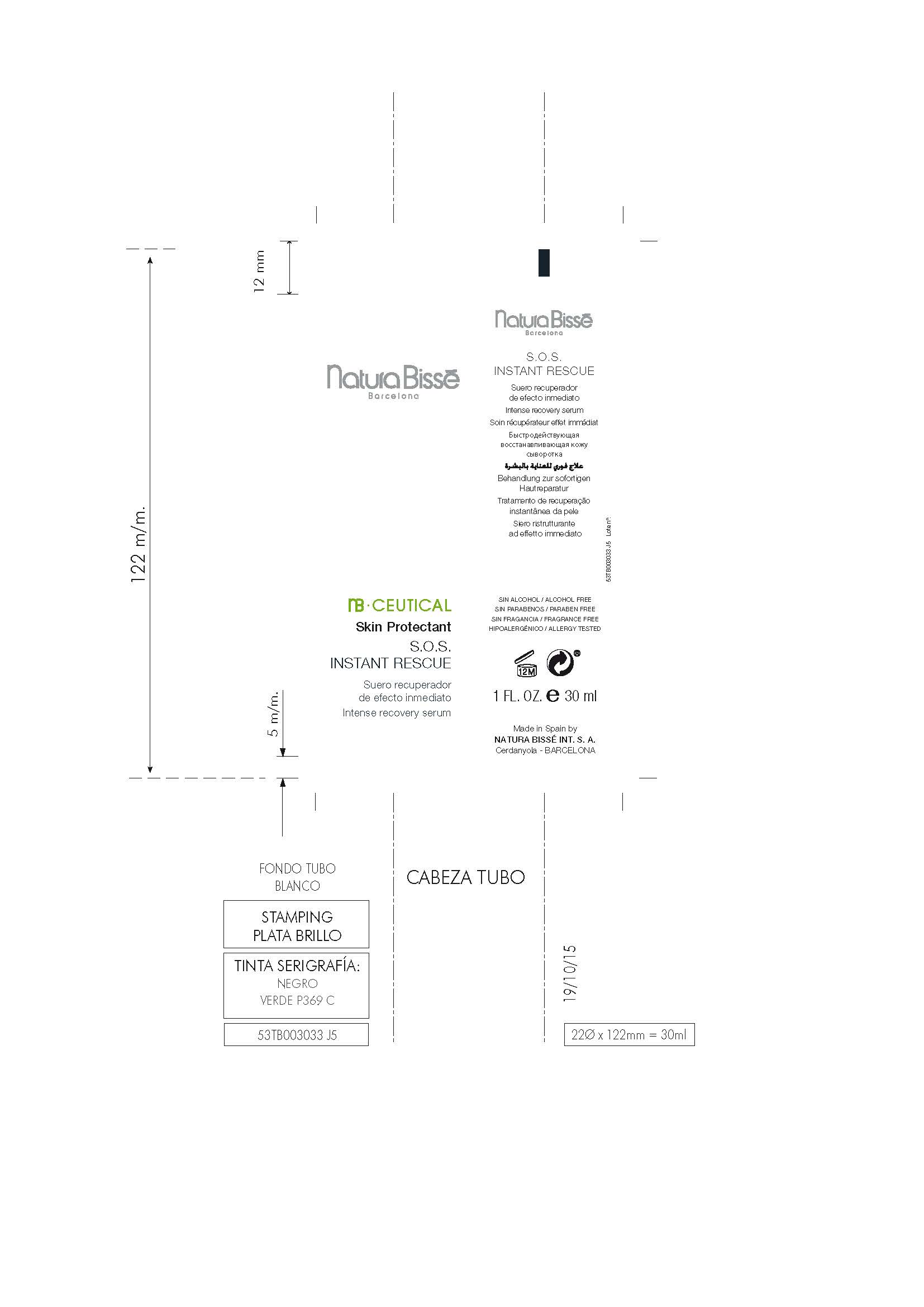
INDICATIONS AND USAGE
Helps prevent, temporarily protects and helps relieve chapped or cracked skin.
Helps prevent and protect from the drying effects of wind and cold weather
INACTIVE INGREDIENTS
Water, Pentylene Glycol, Calendula Officinalis Flower Extract, Methyl Gluceth-10,
Propylene Glycol, Butylene Glycol, Aloe Barbadensis Leaf Extract, Sodium Acrylates
Copolymer, Panthenol, Allantoin, Lecithin, Glycerin, Arginine, Laureth-3, Arnica
Montana Flower Extract, Hydroxyethylcellulose, Propolis Extract, Disodium EDTA,
Potassium Sorbate, Acetyl Dipeptide-1 Cetyl Ester, Urea, Lactic Acid, Sodium Lactate,
Serine, Sorbitol, Pseudoalteromonas Ferment Extract, Sodium Benzoate, Hydrolyzed
Wheat Protein, Sodium Chloride, Hydrolyzed Soy Protein, Ascorbic Acid, Phenoxyethanol,
Caprylyl Glycol, Xanthan Gum, Citric Acid, Tripeptide-10 Citrulline, Carbomer,
Tripeptide-1, Triethanolamine.
WARNINGS
For external use only
When using this product - do not get into eyes.
Do not use on deep or puncture wounds - animal bites - serious burns.
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison
Control Center right away