Label: ANTACID EXTRA STRENGTH- calcium carbonate tablet, chewable
-
NDC Code(s):
11673-479-03,
11673-480-03,
11673-726-01,
11673-726-02, view more11673-726-03
- Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
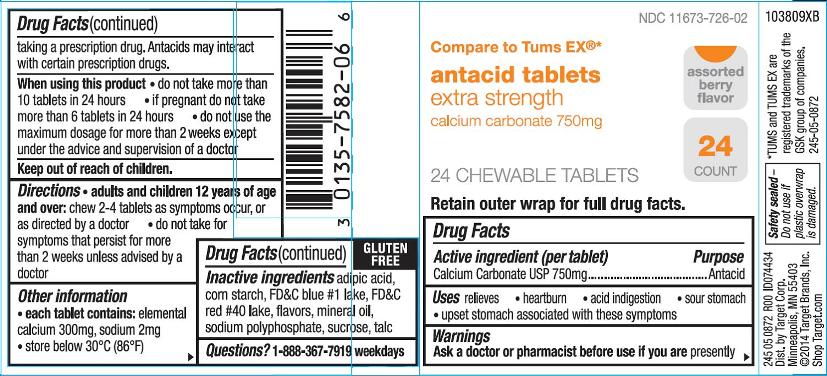
- Active ingredient (per tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug. Antacids may interact with certain prescription drugs.
- Directions
- Other information
- Inactive ingredients (Assorted Berry)
- Inactive ingredients (Assorted Tropical Fruit)
- Questions?
-
Principal Display Panel
NDC 11673-479-03
extra strength
antacid 750
calcium carbonate
Compare to Tums ®Extra Strength*
relief of heartburn
acid indigestion and
upset stomach
associated with
these symptoms
up&up ®
ASSORTED BERRY FLAVOR
96 CHEWABLE TABLETS
artificially flavored
Safety sealed – Do not use if printed inner seal beneath cap is missing or broken.
*TUMS ®is a registered trademark of the GSK group of companies.
245 05 0459 R00 ID285492
Distributed by Target Corporation
Minneapolis, MN 55403
©2015 Target Brands, Inc.
Shop Target.com
GLUTEN FREE
103741XB (front label)
103740XB (back label)
-
Principal Display Panel
NDC 11673-480-03
extra strength
antacid 750
calcium carbonate
Compare to Tums ®Extra Strength*
relief of heartburn
acid indigestion and
upset stomach
associated with
these symptoms
up&up ®
TROPICAL FRUIT FLAVOR
96 CHEWABLE TABLETS
Naturally and artificially flavored
Safety sealed – Do not use if printed inner seal beneath cap is missing or broken.
*TUMS ®is a registered trademark of the GSK group of companies.
245 05 0458 R00 ID285492
Distributed by Target Corporation
Minneapolis, MN 55403
©2015 Target Brands, Inc.
Shop Target.com
GLUTEN FREE
103745XB (front label)
103744XB (back label)
-
Principal Display Panel
NDC 11673-726-02
antacid tablets
extra strength
calcium carbonate 750mg
Compare to Tums EX ®*
Assorted berry flavor
24 count
24 CHEWABLE TABLETS
Retain outer wrap for full drug facts.
GLUTEN FREE
245 05 0872 R00 ID074434
Dist. By Target Corp.
Minneapolis, MN 55403
©2014 Target Brands, Inc.
Shop Target.com
Safety sealed-Do not use if plastic overwrap is damaged.
*TUMS and TUMS EX are registered trademarks of the GSK group of companies.
245-05-0872
103809XB
-
INGREDIENTS AND APPEARANCE
ANTACID EXTRA STRENGTH
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-479 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D, CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) MINERAL OIL (UNII: T5L8T28FGP) ADIPIC ACID (UNII: 76A0JE0FKJ) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color pink (mauve, bluish) Score no score Shape ROUND Size 16mm Flavor STRAWBERRY (Assorted Berry, raspberry, mixed berry) Imprint Code 3205 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-479-03 96 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 02/15/2010 ANTACID EXTRA STRENGTH
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-480 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D, CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) MINERAL OIL (UNII: T5L8T28FGP) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color red (red-orange, orange, off-white, light yellow) Score no score Shape ROUND Size 16mm Flavor TROPICAL FRUIT PUNCH (Assorted tropical fruit flavor, tropical punch, mandarin orange, orange-pineapple, strawberry-banana) Imprint Code 3205 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-480-03 96 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 02/15/2010 ANTACID EXTRA STRENGTH
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-726 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D, CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) MINERAL OIL (UNII: T5L8T28FGP) ADIPIC ACID (UNII: 76A0JE0FKJ) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color pink (mauve, bluish) Score no score Shape ROUND Size 16mm Flavor STRAWBERRY (Assorted Berry, raspberry, mixed berry) Imprint Code 3205 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-726-02 3 in 1 CELLO PACK 03/10/2014 1 NDC:11673-726-01 8 in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC:11673-726-03 200 in 1 BOTTLE; Type 0: Not a Combination Product 03/10/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 03/10/2014 Labeler - Target Corporation (006961700)