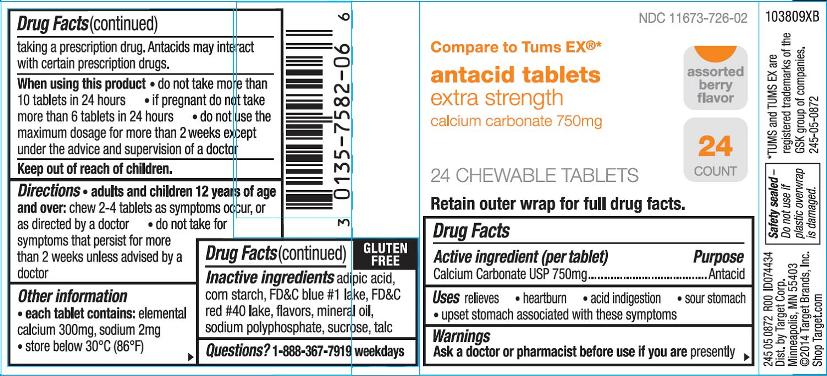
Warnings
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug. Antacids may interact with certain prescription drugs.
Directions
- adults and children 12 years of age and over:chew 2 – 4 tablets as symptoms occur, or as directed by a doctor
- do not take for symptoms that persist for more than 2 weeks unless advised by a doctor
Other information
- each tablet contains:elemental calcium 300mg, sodium 2mg
- store below 30 oC (86 oF)
Inactive ingredients (Assorted Berry)
adipic acid, corn starch, FD&C blue #1 lake, FD&C red #40 lake, flavors, mineral oil, sodium polyphosphate, sucrose, talc
Inactive ingredients (Assorted Tropical Fruit)
corn startch, FD&C red #40 lake, FD&C yellow #5(tartrazine) lake, FD&C yellow #6 lake, flavors, mineral oil, sodium polyphosphate, sucrose, talc
Principal Display Panel
NDC 11673-479-03
extra strength
antacid 750
calcium carbonate
Compare to Tums ®Extra Strength*
relief of heartburn
acid indigestion and
upset stomach
associated with
these symptoms
up&up ®
ASSORTED BERRY FLAVOR
96 CHEWABLE TABLETS
artificially flavored
Safety sealed – Do not use if printed inner seal beneath cap is missing or broken.
*TUMS ®is a registered trademark of the GSK group of companies.
245 05 0459 R00 ID285492
Distributed by Target Corporation
Minneapolis, MN 55403
©2015 Target Brands, Inc.
Shop Target.com
GLUTEN FREE
103741XB (front label)
103740XB (back label)
Principal Display Panel
NDC 11673-480-03
extra strength
antacid 750
calcium carbonate
Compare to Tums ®Extra Strength*
relief of heartburn
acid indigestion and
upset stomach
associated with
these symptoms
up&up ®
TROPICAL FRUIT FLAVOR
96 CHEWABLE TABLETS
Naturally and artificially flavored
Safety sealed – Do not use if printed inner seal beneath cap is missing or broken.
*TUMS ®is a registered trademark of the GSK group of companies.
245 05 0458 R00 ID285492
Distributed by Target Corporation
Minneapolis, MN 55403
©2015 Target Brands, Inc.
Shop Target.com
GLUTEN FREE
103745XB (front label)
103744XB (back label)
Principal Display Panel
NDC 11673-726-02
antacid tablets
extra strength
calcium carbonate 750mg
Compare to Tums EX ®*
Assorted berry flavor
24 count
24 CHEWABLE TABLETS
Retain outer wrap for full drug facts.
GLUTEN FREE
245 05 0872 R00 ID074434
Dist. By Target Corp.
Minneapolis, MN 55403
©2014 Target Brands, Inc.
Shop Target.com
Safety sealed-Do not use if plastic overwrap is damaged.
*TUMS and TUMS EX are registered trademarks of the GSK group of companies.
245-05-0872
103809XB