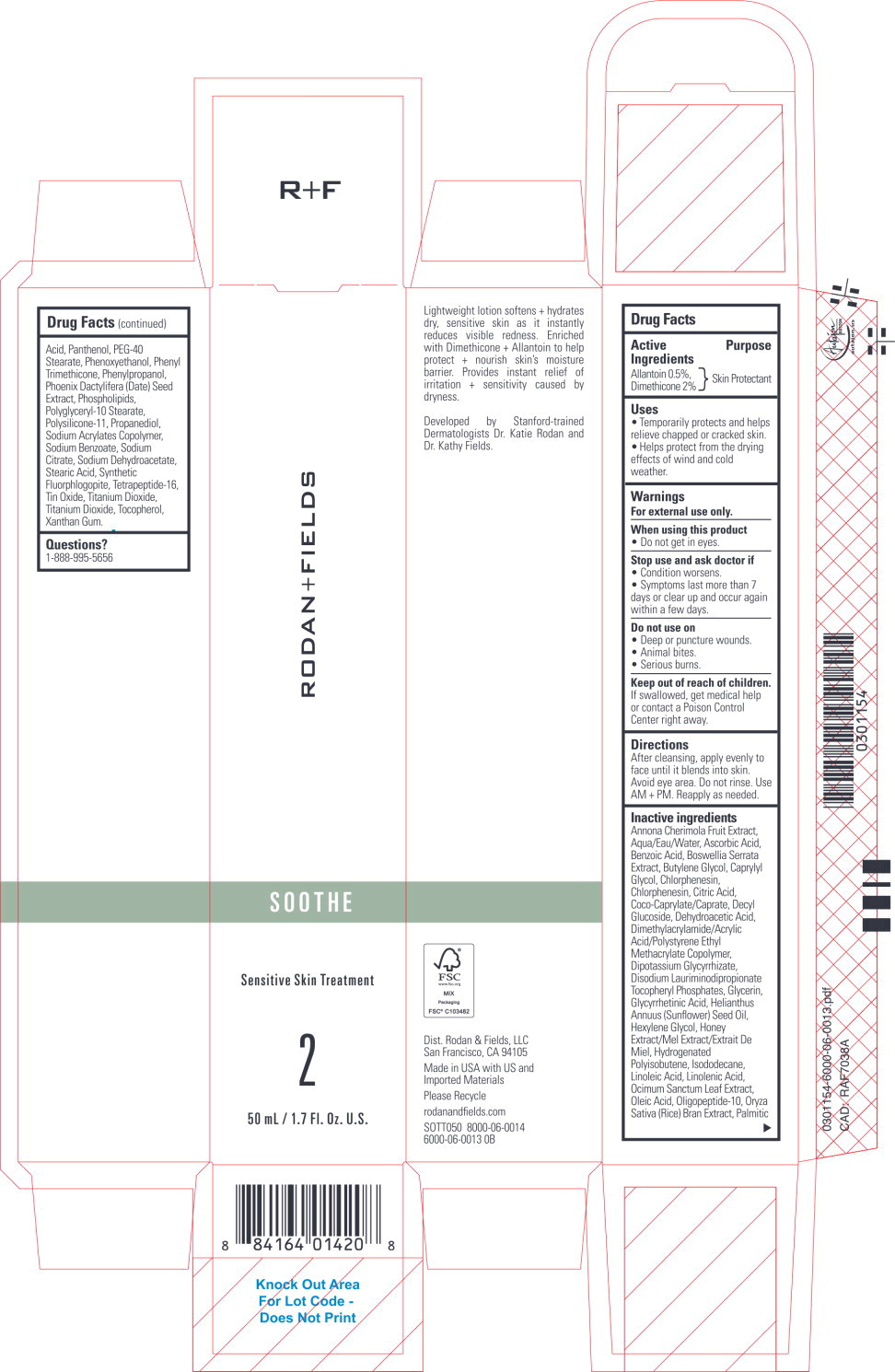
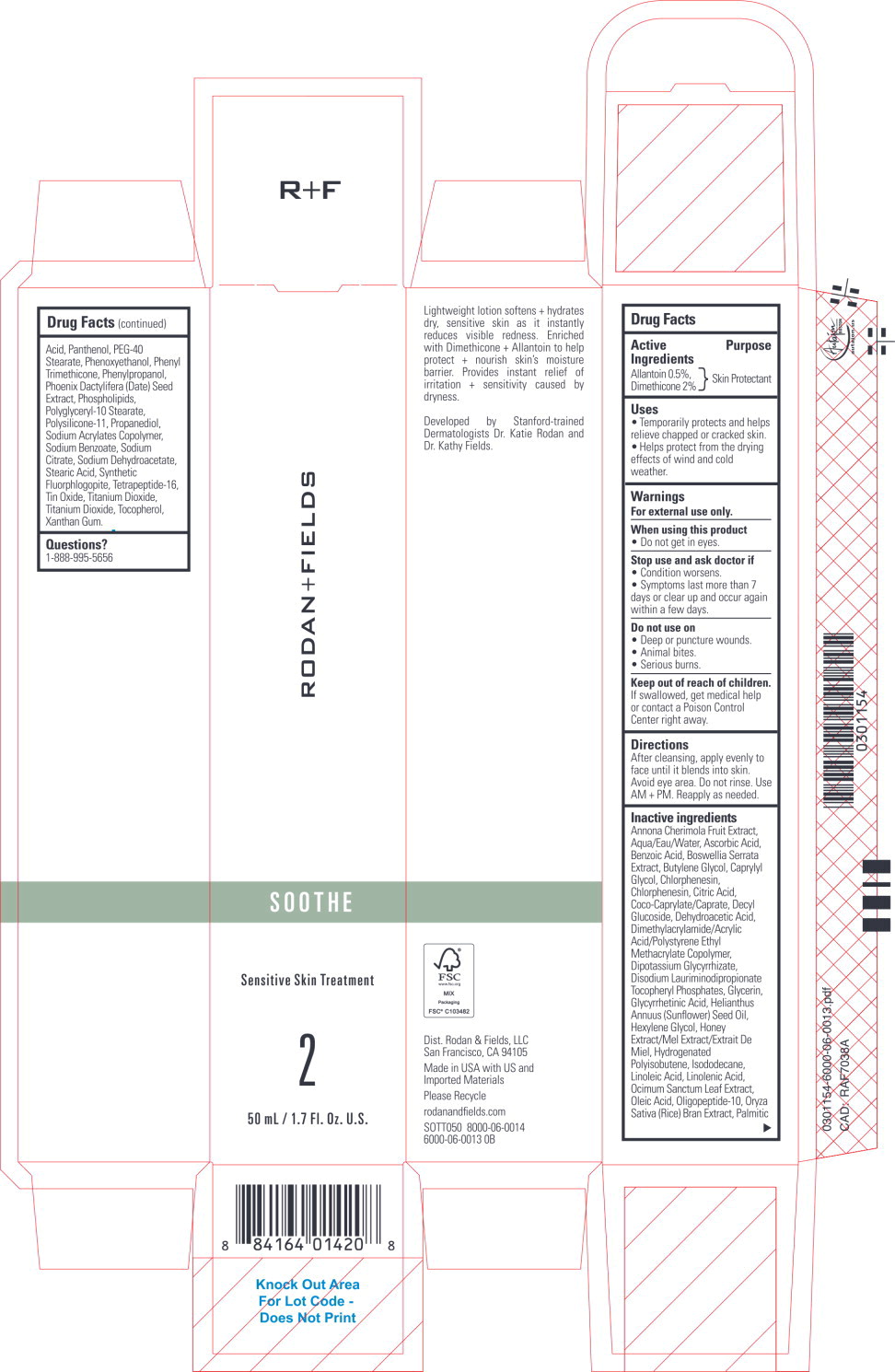
Label: SOOTHE SENSITIVE SKIN TREATMENT- allantoin, dimethicone lotion
- NDC Code(s): 14222-2420-1, 14222-2420-2, 14222-2420-3
- Packager: Rodan & Fields
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Annona Cherimola Fruit Extract, Aqua/Eau/Water, Ascorbic Acid, Benzoic Acid, Boswellia Serrata Extract, Butylene Glycol, Caprylyl Glycol, Chlorphenesin, Chlorphenesin, Citric Acid, Coco-Caprylate/Caprate, Decyl Glucoside, Dehydroacetic Acid, Dimethylacrylamide/Acrylic Acid/Polystyrene Ethyl Methacrylate Copolymer, Dipotassium Glycyrrhizate, Disodium Lauriminodipropionate Tocopheryl Phosphates, Glycerin, Glycyrrhetinic Acid, Helianthus Annuus (Sunflower) Seed Oil, Hexylene Glycol, Honey Extract/Mel Extract/Extrait De Miel, Hydrogenated Polyisobutene, Isododecane, Linoleic Acid, Linolenic Acid, Ocimum Sanctum Leaf Extract, Oleic Acid, Oligopeptide-10, Oryza Sativa (Rice) Bran Extract, Palmitic Acid, Panthenol, PEG-40 Stearate, Phenoxyethanol, Phenyl Trimethicone, Phenylpropanol, Phoenix Dactylifera (Date) Seed Extract, Phospholipids, Polyglyceryl-10 Stearate, Polysilicone-11, Propanediol, Sodium Acrylates Copolymer, Sodium Benzoate, Sodium Citrate, Sodium Dehydroacetate, Stearic Acid, Synthetic Fluorphlogopite, Tetrapeptide-16, Tin Oxide, Titanium Dioxide, Titanium Dioxide, Tocopherol, Xanthan Gum.
- Questions?
- Principal Display Panel – 50 mL Carton Label
- Principal Display Panel – 50 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
SOOTHE SENSITIVE SKIN TREATMENT
allantoin, dimethicone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Allantoin (UNII: 344S277G0Z) (Allantoin - UNII:344S277G0Z) Allantoin 0.5 g in 100 mL Dimethicone (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) Dimethicone 2 g in 100 mL Inactive Ingredients Ingredient Name Strength CHERIMOYA (UNII: 33WVT714QS) ASCORBIC ACID (UNII: PQ6CK8PD0R) BENZOIC ACID (UNII: 8SKN0B0MIM) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) DEHYDROACETIC ACID (UNII: 2KAG279R6R) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) DISODIUM LAURIMINODIPROPIONATE TOCOPHERYL PHOSPHATES (UNII: 0K5Y9U1P6M) GLYCERIN (UNII: PDC6A3C0OX) ENOXOLONE (UNII: P540XA09DR) SUNFLOWER OIL (UNII: 3W1JG795YI) HEXYLENE GLYCOL (UNII: KEH0A3F75J) HONEY (UNII: Y9H1V576FH) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) ISODODECANE (UNII: A8289P68Y2) LINOLEIC ACID (UNII: 9KJL21T0QJ) LINOLENIC ACID (UNII: 0RBV727H71) HOLY BASIL LEAF (UNII: SCJ765569P) OLEIC ACID (UNII: 2UMI9U37CP) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PALMITIC ACID (UNII: 2V16EO95H1) PANTHENOL (UNII: WV9CM0O67Z) PEG-40 STEARATE (UNII: ECU18C66Q7) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PHENYLPROPANOL (UNII: 0F897O3O4M) PHOENIX DACTYLIFERA SEED (UNII: 73NE6T0Q00) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-10 STEARATE (UNII: 90TF85HH91) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PROPANEDIOL (UNII: 5965N8W85T) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) STANNIC OXIDE (UNII: KM7N50LOS6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2420-1 1 in 1 CARTON 03/09/2021 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:14222-2420-2 1 in 1 CARTON 06/01/2021 2 10 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:14222-2420-3 2 mL in 1 PACKET; Type 0: Not a Combination Product 04/12/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 03/09/2021 Labeler - Rodan & Fields (051659584)