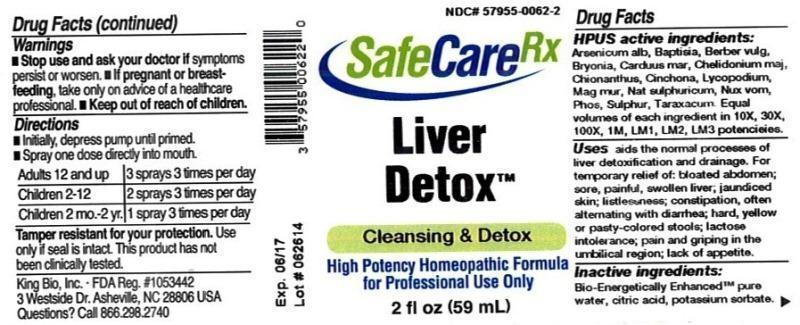
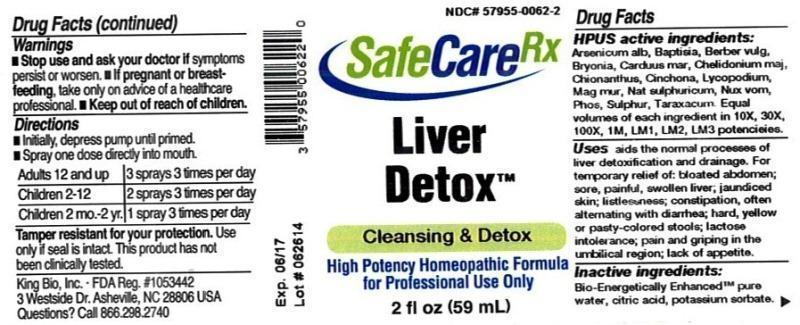
Label: LIVER DETOX- arsenicum album, baptisia tinctoria, barberis vulgaris, bryonia, carduus marianus, chelidonium majus, chionanthus virginica, cinchona officinalis, lycopodium clavatum, magnesia muriatica, natrum sulphuricum, nux vomica, phosphorus, sulphur, taraxacum officinale liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-0062-2 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 23, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Drug Facts__________________________________________________________________________________________________________
HPUS active ingredients: Arsenicum album, Baptisia tinctoria, Berberis vulgaris, Bryonia, Carduus marianus, Chelidonium majus, Chionanthus virginica, Cinchona officinalis, Lycopodium clavatum, Magnesia muriatica, Natrum sulphuricum, Nux vomica, Phosphorus, Sulphur, Taraxacum officinale. Equal volumes of each ingredient in 10X, 30X, 100X, 1M, LM1, LM2, LM3 potencies.
-
INDICATIONS & USAGE
Uses aids the normal processes of liver detoxification and drainage. For temporary relief of: bloated abdomen; sore, painful, swollen liver; jaundiced skin; listlessness; constipation, often alternating with diarrhea; hard, yellow or pasty-colored stools; lactose intolerance; pain and griping in the umbilical region; lack of appetite.
- INACTIVE INGREDIENT
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
PURPOSE
Uses aids the normal processes of liver detoxification and drainage. For temporary relief of:
- bloated abdomen
- sore, painful, swollen liver
- jaundiced skin
- listlessness; constipation
- often alternating with diarrhea; hard, yellow or pasty-colored stools
- lactose intolerance
- pain and griping in the umbilical region
- lack of appetite
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIVER DETOX
arsenicum album, baptisia tinctoria, barberis vulgaris, bryonia, carduus marianus, chelidonium majus, chionanthus virginica, cinchona officinalis, lycopodium clavatum, magnesia muriatica, natrum sulphuricum, nux vomica, phosphorus, sulphur, taraxacum officinale liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-0062 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 10 [hp_X] in 59 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 10 [hp_X] in 59 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 10 [hp_X] in 59 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 10 [hp_X] in 59 mL MILK THISTLE (UNII: U946SH95EE) (MILK THISTLE - UNII:U946SH95EE) MILK THISTLE 10 [hp_X] in 59 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 10 [hp_X] in 59 mL CHIONANTHUS VIRGINICUS BARK (UNII: S9Y4B22U2E) (CHIONANTHUS VIRGINICUS BARK - UNII:S9Y4B22U2E) CHIONANTHUS VIRGINICUS BARK 10 [hp_X] in 59 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 10 [hp_X] in 59 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 10 [hp_X] in 59 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 10 [hp_X] in 59 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 10 [hp_X] in 59 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 10 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 10 [hp_X] in 59 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 10 [hp_X] in 59 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-0062-2 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/03/2012 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(57955-0062)