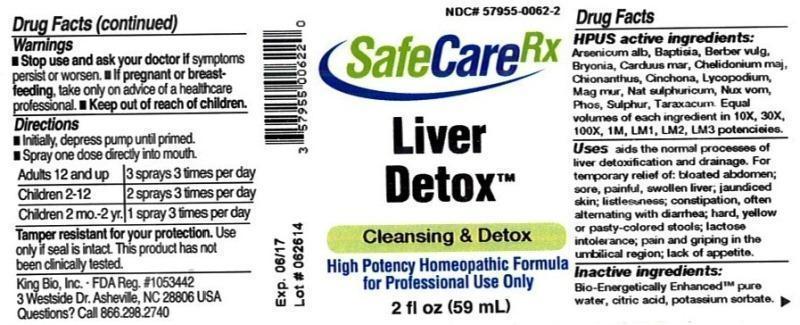
Drug Facts__________________________________________________________________________________________________________
HPUS active ingredients: Arsenicum album, Baptisia tinctoria, Berberis vulgaris, Bryonia, Carduus marianus, Chelidonium majus, Chionanthus virginica, Cinchona officinalis, Lycopodium clavatum, Magnesia muriatica, Natrum sulphuricum, Nux vomica, Phosphorus, Sulphur, Taraxacum officinale. Equal volumes of each ingredient in 10X, 30X, 100X, 1M, LM1, LM2, LM3 potencies.
Uses aids the normal processes of liver detoxification and drainage. For temporary relief of: bloated abdomen; sore, painful, swollen liver; jaundiced skin; listlessness; constipation, often alternating with diarrhea; hard, yellow or pasty-colored stools; lactose intolerance; pain and griping in the umbilical region; lack of appetite.
Warnings
- Stop use and ask your doctor if symptoms persist or worsen.
- If pregnant or breast-feeding, take only on advice of a healthcare professional.
Directions
- Initially, depress pump until primed.
- Spray one dose directly into mouth.
- Adults 12 and up: 3 sprays 3 times per day
- Children 2-12: 2 sprays 3 times per day
- Children 2 mo.-2 yr.: 1 spray 3 times per day
Tamper resistant for your protection. Use only if safety seal is intact. This product has not been clinically tested.
Uses aids the normal processes of liver detoxification and drainage. For temporary relief of:
- bloated abdomen
- sore, painful, swollen liver
- jaundiced skin
- listlessness; constipation
- often alternating with diarrhea; hard, yellow or pasty-colored stools
- lactose intolerance
- pain and griping in the umbilical region
- lack of appetite