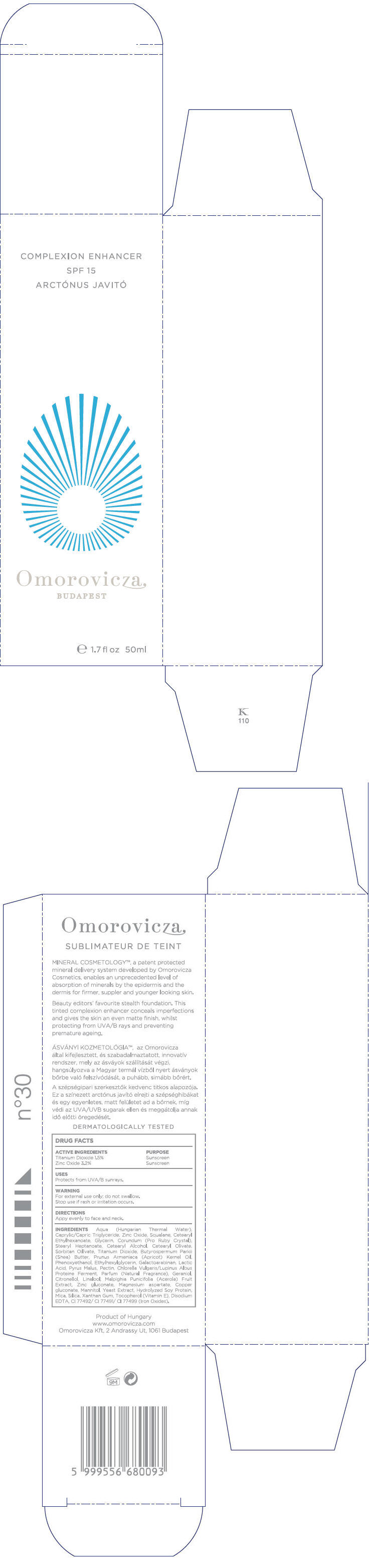
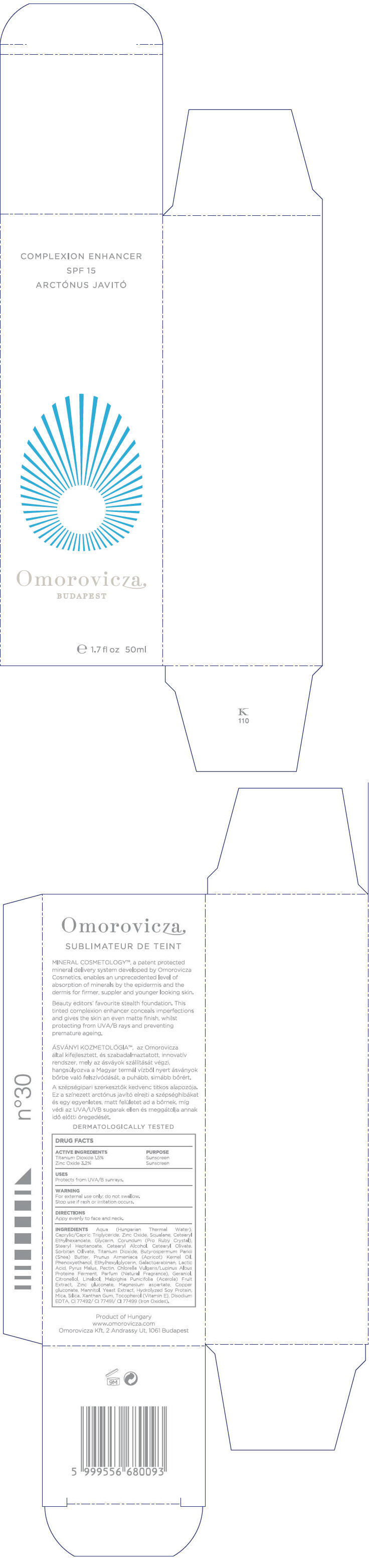
Label: COMPLEXION ENHANCER SPF15- titanium dioxide and zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 41442-110-01, 41442-110-02 - Packager: Omorovicza Kozmetikai Kft.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 1, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- USES
- WARNING
- DIRECTIONS
-
INGREDIENTS
Aqua (Hungarian Thermal Water), Caprylic/Capric Triglyceride, Zinc Oxide, Squalane, Cetearyl Ethylhexanoate, Glycerin, Corundum (Pro Ruby Crystal), Stearyl Heptanoate, Cetearyl Alcohol, Cetearyl Olivate, Sorbitan Olivate, Titanium Dioxide, Butyrospermum Parkii (Shea) Butter, Prunus Armeniaca (Apricot) Kernel Oil, Phenoxyethanol, Ethylhexylglycerin, Galactoarabinan, Lactic Acid, Pyrus Malus, Pectin, Chlorella Vulgaris/Lupinus Albus Protein Ferment, Parfum (Fragrance), Geraniol, Citronellol, Linalool, Malpighia Punicifolia (Acerola) Fruit Extract, Zinc gluconate, Magnesium aspartate, Copper gluconate, Mannitol, Yeast Extract, Hydrolyzed Soy Protein, Mica, Silica, Xanthan Gum, Tocopherol (Vitamin E), Disodium EDTA, CI 77492/ CI 77491/ CI 77499 (Iron Oxides)
- PRINCIPAL DISPLAY PANEL - 50ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
COMPLEXION ENHANCER SPF15
titanium dioxide and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41442-110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium dioxide (UNII: 15FIX9V2JP) (Titanium dioxide - UNII:15FIX9V2JP) Titanium dioxide 0.75 mL in 50 mL Zinc oxide (UNII: SOI2LOH54Z) (Zinc oxide - UNII:SOI2LOH54Z) Zinc oxide 1.6 mL in 50 mL Inactive Ingredients Ingredient Name Strength Caprylic/Capric Mono/Diglycerides (UNII: U72Q2I8C85) Squalane (UNII: GW89575KF9) Cetearyl Ethylhexanoate (UNII: 9M64UO4C25) Glycerin (UNII: PDC6A3C0OX) Aluminum Oxide (UNII: LMI26O6933) Stearyl Heptanoate (UNII: 2M4UGL1NCN) Cetostearyl Alcohol (UNII: 2DMT128M1S) Cetearyl Olivate (UNII: 58B69Q84JO) Sorbitan Olivate (UNII: MDL271E3GR) Shea Butter (UNII: K49155WL9Y) Apricot Seed Oil (UNII: 54JB35T06A) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylhexylglycerin (UNII: 147D247K3P) Lactic Acid (UNII: 33X04XA5AT) Apple (UNII: B423VGH5S9) Pectin (UNII: 89NA02M4RX) Geraniol (UNII: L837108USY) .BETA.-CITRONELLOL, (+/-)- (UNII: 565OK72VNF) LINALOOL, (+)- (UNII: F4VNO44C09) Acerola (UNII: XDD2WEC9L5) Zinc Gluconate (UNII: U6WSN5SQ1Z) Magnesium Aspartate (UNII: R17X820ROL) Copper Gluconate (UNII: RV823G6G67) Mannitol (UNII: 3OWL53L36A) Yeast (UNII: 3NY3SM6B8U) Soy Protein (UNII: R44IWB3RN5) Mica (UNII: V8A1AW0880) Silicon Dioxide (UNII: ETJ7Z6XBU4) Xanthan Gum (UNII: TTV12P4NEE) Alpha-Tocopherol (UNII: H4N855PNZ1) Edetate Disodium (UNII: 7FLD91C86K) Ferrosoferric Oxide (UNII: XM0M87F357) Product Characteristics Color BROWN Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41442-110-01 1 in 1 BOX 1 50 mL in 1 BOTTLE, GLASS 2 NDC:41442-110-02 125 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/01/2011 Labeler - Omorovicza Kozmetikai Kft. (525432105) Establishment Name Address ID/FEI Business Operations Omorovicza Kozmetikai Kft. 525432105 LABEL, MANUFACTURE