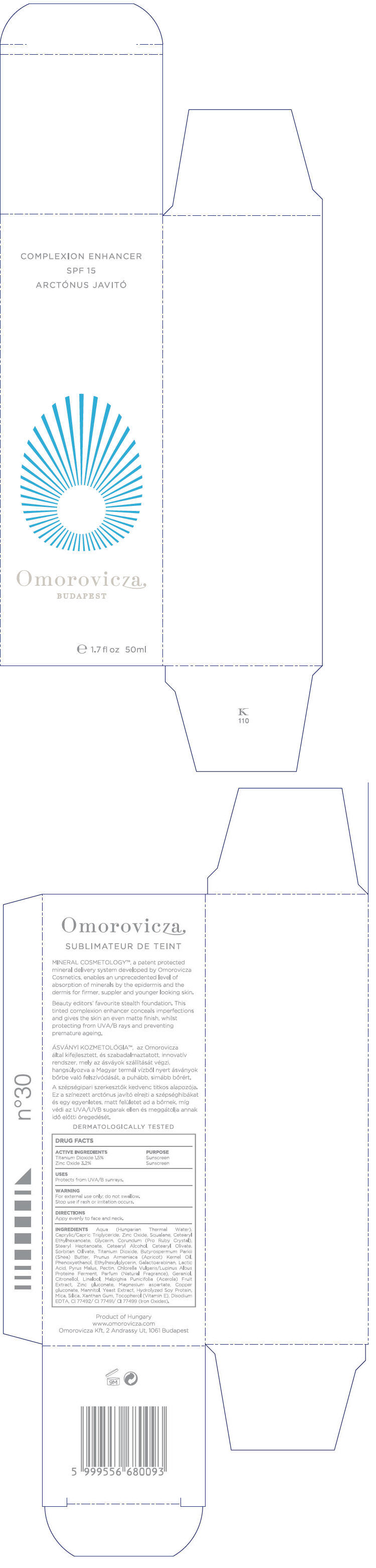
INGREDIENTS
Aqua (Hungarian Thermal Water), Caprylic/Capric Triglyceride, Zinc Oxide, Squalane, Cetearyl Ethylhexanoate, Glycerin, Corundum (Pro Ruby Crystal), Stearyl Heptanoate, Cetearyl Alcohol, Cetearyl Olivate, Sorbitan Olivate, Titanium Dioxide, Butyrospermum Parkii (Shea) Butter, Prunus Armeniaca (Apricot) Kernel Oil, Phenoxyethanol, Ethylhexylglycerin, Galactoarabinan, Lactic Acid, Pyrus Malus, Pectin, Chlorella Vulgaris/Lupinus Albus Protein Ferment, Parfum (Fragrance), Geraniol, Citronellol, Linalool, Malpighia Punicifolia (Acerola) Fruit Extract, Zinc gluconate, Magnesium aspartate, Copper gluconate, Mannitol, Yeast Extract, Hydrolyzed Soy Protein, Mica, Silica, Xanthan Gum, Tocopherol (Vitamin E), Disodium EDTA, CI 77492/ CI 77491/ CI 77499 (Iron Oxides)