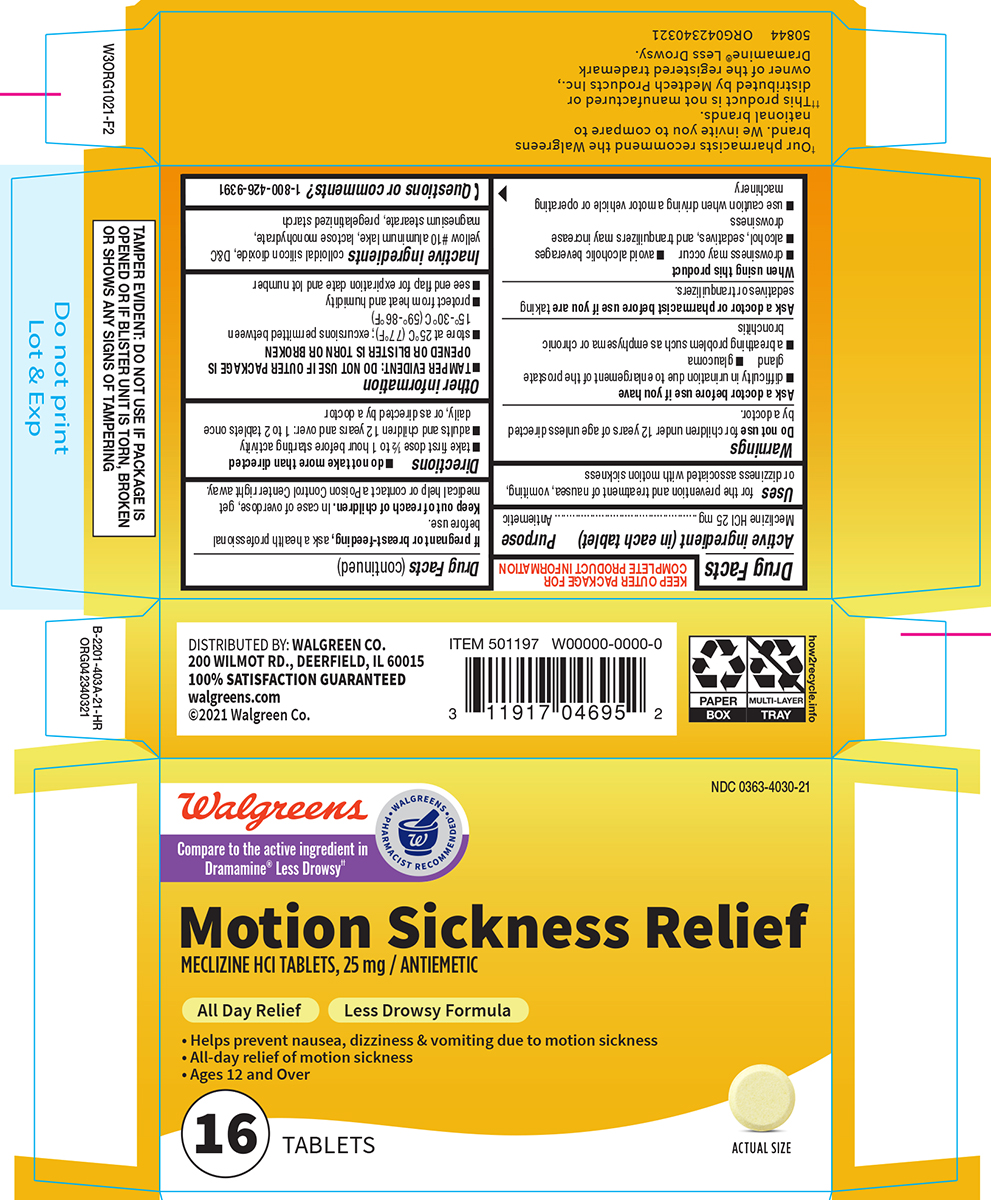
Label: MOTION SICKNESS RELIEF- meclizine hcl tablet
- NDC Code(s): 0363-4030-21
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
Walgreens
• WALGREENS •
PHARMACIST RECOMMENDED†Compare to the active ingredient in
Dramamine® Less Drowsy††NDC 0363-4030-21
Motion Sickness Relief
MECLIZINE HCl TABLETS, 25 mg / ANTIEMETICAll Day Relief Less Drowsy Formula
• Helps prevent nausea, dizziness & vomiting due to motion sickness
• All-day relief of motion sickness
• Ages 12 and Over16 TABLETS
ACTUAL SIZE
†Our pharmacists recommend the Walgreens
brand. We invite you to compare to
national brands.
††This product is not manufactured or
distributed by Medtech Products Inc.,
owner of the registered trademark
Dramamine® Less Drowsy.50844 ORG042340321
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERINGDISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
walgreens.com
©2021 Walgreen Co.
Walgreens 44-403
-
INGREDIENTS AND APPEARANCE
MOTION SICKNESS RELIEF
meclizine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-4030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color yellow Score no score Shape ROUND Size 9mm Flavor Imprint Code 44;403 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-4030-21 2 in 1 CARTON 10/21/2021 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 10/21/2021 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(0363-4030) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(0363-4030) , pack(0363-4030) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(0363-4030) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(0363-4030)