Label: ASPERCREME- lidocaine patch
- NDC Code(s): 62168-0584-4, 62168-0584-5, 62168-0584-6
- Packager: Lead Chemical Co. Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 25, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient – Lidocaine Patch US 1ct
- Purpose – Lidocaine Patch US 1ct
- Use – Lidocaine Patch US 1ct
-
Warnings – Lidocaine Patch US 1ct
For external use only
Do not use – Lidocaine Patch US 1ct
- •
- on wounds or on irritated or damage skin
- •
- more than 1 patch on your body at a time or with other topical analgesics at the same time
- •
- with a heating pad
When using this product – Lidocaine Patch US 1ct
- •
- use only as directed
- •
- do not bandage tightly
- •
- avoid contact with the eyes and mucous membranes
- •
- do not expose the area to local heat or to direct sunlight
Stop use and ask a doctor if – Lidocaine Patch US 1ct
- •
- condition worsens or symptoms persist for more than 7 days
- •
- symptoms clear up and occur again within a few days
- •
- redness is present or excessive skin irritation occurs
- •
- you experience severe burning pain, swelling, or blistering where the product was applied
-
Directions – Lidocaine Patch US 1ct
• adults and children 12 years of age and older:
- •
- clean and dry affected area
- •
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- •
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- •
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- •
- apply to the affected area not more than 3 to 4 times daily
- •
- wash hands after applying or removing patch. Throw away the patch after folding sticky ends together.
• children under 12 years of age: ask a doctor
- Inactive ingredients – Lidocaine Patch US 1ct
- Active ingredient – Lidocaine Patch Singles US 1ct
- Purpose – Lidocaine Patch Singles US 1ct
- Use – Lidocaine Patch Singles US 1ct
-
Warnings – Lidocaine Patch Singles US 1ct
For external use only
Do not use – Lidocaine Patch Singles US 1ct
- •
- on puncture wounds, cuts, irritated or swollen skin
- •
- more than 1 patch on your body at a time or with other topical analgesics at the same time
- •
- with a heating pad or apply local heat to the area of use
When using this product – Lidocaine Patch Singles US 1ct
- •
- use only as directed
- •
- do not bandage tightly
- •
- avoid contact with the eyes
- •
- dispose of used patch in manner that always keeps product away from children or pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
Stop use and ask a doctor if – Lidocaine Patch Singles US 1ct
- •
- condition worsens or symptoms persist for more than 7 days
- •
- symptoms clear up and occur again within a few days
- •
- redness or irritation develops
- •
- you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
-
Directions – Lidocaine Patch Singles US 1ct
adults and children over 12 years of age and older:
- •
- clean and dry affected area
- •
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- •
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- •
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- •
- use 1 patch at a time and not more than 3 to 4 times daily
children under 12 years of age: consult a doctor
- Inactive ingredients – Lidocaine Patch Singles US 1ct
-
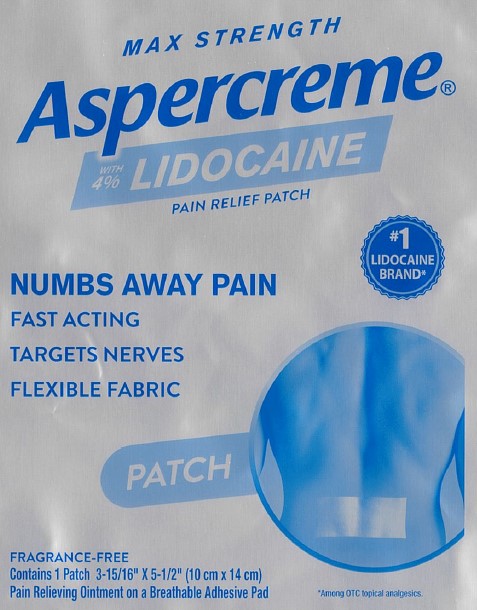
Package/Label Principal Display Panel – Lidocaine Patch US 1ct
MAX STRENGTH
Aspercreme®with 4% LIDOCAINE
PAIN RELIEF PATCHNUMBS AWAY PAINFAST ACTING
TARGETS NERVES
FLEXIBLE FABRIC
#1 LIDOCAINE BRAND*PATCH
FRAGRANCE-FREE
Contains 1 Patch 3-15/16” x 5-1/2” (10 cm x 14 cm)
Pain Relieving Ointment on a Breathable Adhesive Pad*Among OTC topical analgesics.
Dist. by Chattem, Inc., a Sanofi Company
P.O. Box 2219, Chattanooga, TN
37409-0219 USA
©2022
www.aspercreme.com Made in Japan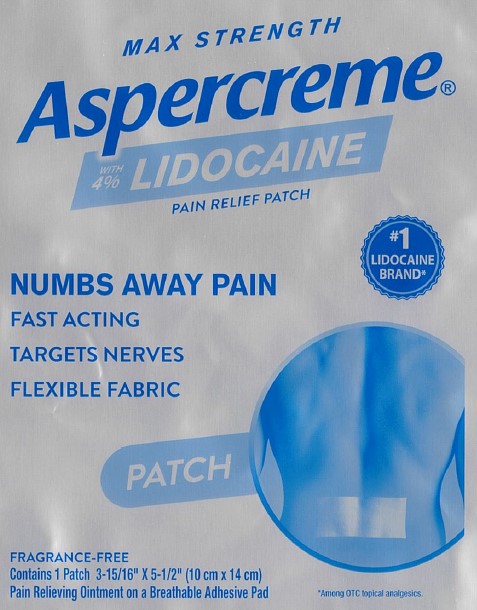
Label
-
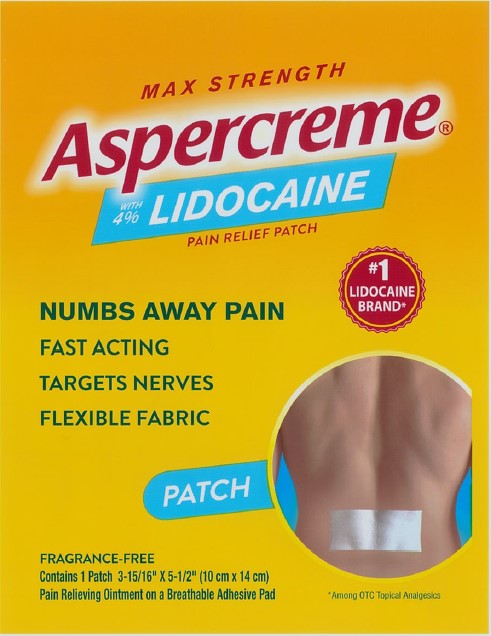
Package/Label Principal Display Panel – Lidocaine Patch Singles US 1ct
MAX STRENGTH
Aspercreme®with 4% LIDOCAINE
PAIN RELIEF PATCHNUMBS AWAY PAINFAST ACTING
TARGETS NERVES
FLEXIBLE FABRIC
#1 LIDOCAINE BRAND*PATCH
FRAGRANCE-FREE
Contains 1 Patch 3-15/16” x 5-1/2” (10 cm x 14 cm)
Pain Relieving Ointment on a Breathable Adhesive Pad*Among OTC topical analgesics
CHATTEM®
A SANOFI COMPANY
Dist. by Chattem, Inc., a Sanofi Company
P.O. Box 2219, Chattanooga, TN
37409-0219 USA ©2020
www.aspercreme.com
Made in Japan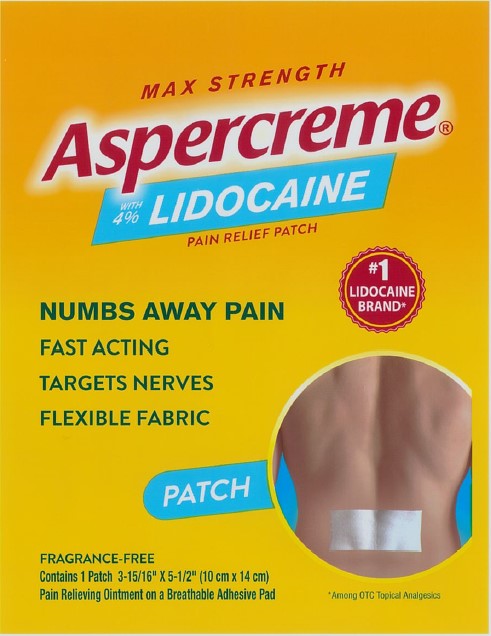
Label
-
INGREDIENTS AND APPEARANCE
ASPERCREME
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62168-0584 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 246 mg Inactive Ingredients Ingredient Name Strength DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) NONOXYNOL-30 (UNII: JJX07DG188) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62168-0584-4 400 in 1 CARTON 05/17/2021 03/31/2025 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:62168-0584-5 400 in 1 CARTON 02/02/2022 2 1 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:62168-0584-6 400 in 1 CARTON 07/15/2023 3 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/16/2016 Labeler - Lead Chemical Co. Ltd. (693727091)