Warnings – Lidocaine Patch US 1ct
For external use only
Do not use – Lidocaine Patch US 1ct
- •
- on wounds or on irritated or damage skin
- •
- more than 1 patch on your body at a time or with other topical analgesics at the same time
- •
- with a heating pad
When using this product – Lidocaine Patch US 1ct
- •
- use only as directed
- •
- do not bandage tightly
- •
- avoid contact with the eyes and mucous membranes
- •
- do not expose the area to local heat or to direct sunlight
Stop use and ask a doctor if – Lidocaine Patch US 1ct
- •
- condition worsens or symptoms persist for more than 7 days
- •
- symptoms clear up and occur again within a few days
- •
- redness is present or excessive skin irritation occurs
- •
- you experience severe burning pain, swelling, or blistering where the product was applied
Directions – Lidocaine Patch US 1ct
• adults and children 12 years of age and older:
- •
- clean and dry affected area
- •
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- •
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- •
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- •
- apply to the affected area not more than 3 to 4 times daily
- •
- wash hands after applying or removing patch. Throw away the patch after folding sticky ends together.
• children under 12 years of age: ask a doctor
Inactive ingredients – Lidocaine Patch US 1ct
aluminum glycinate, aluminum hydroxide, cellulose gum, glycerin, methyl acrylate/2-ethylhexyl acrylate copolymer, methylparaben, nonoxynol-30, polyacrylic acid, polysorbate 80, propylene glycol, silica, sodium polyacrylate, tartaric acid, titanium dioxide, urea, water
Warnings – Lidocaine Patch Singles US 1ct
For external use only
Do not use – Lidocaine Patch Singles US 1ct
- •
- on puncture wounds, cuts, irritated or swollen skin
- •
- more than 1 patch on your body at a time or with other topical analgesics at the same time
- •
- with a heating pad or apply local heat to the area of use
When using this product – Lidocaine Patch Singles US 1ct
- •
- use only as directed
- •
- do not bandage tightly
- •
- avoid contact with the eyes
- •
- dispose of used patch in manner that always keeps product away from children or pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
Stop use and ask a doctor if – Lidocaine Patch Singles US 1ct
- •
- condition worsens or symptoms persist for more than 7 days
- •
- symptoms clear up and occur again within a few days
- •
- redness or irritation develops
- •
- you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
Directions – Lidocaine Patch Singles US 1ct
adults and children over 12 years of age and older:
- •
- clean and dry affected area
- •
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- •
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- •
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- •
- use 1 patch at a time and not more than 3 to 4 times daily
children under 12 years of age: consult a doctor
Inactive ingredients – Lidocaine Patch Singles US 1ct
aluminum glycinate, aluminum hydroxide, cellulose gum, glycerin, methyl acrylate/2-ethylhexyl acrylate copolymer, methylparaben, nonoxynol-30, polyacrylic acid, polysorbate 80, propylene glycol, silica, sodium polyacrylate, tartaric acid, titanium dioxide, urea, water
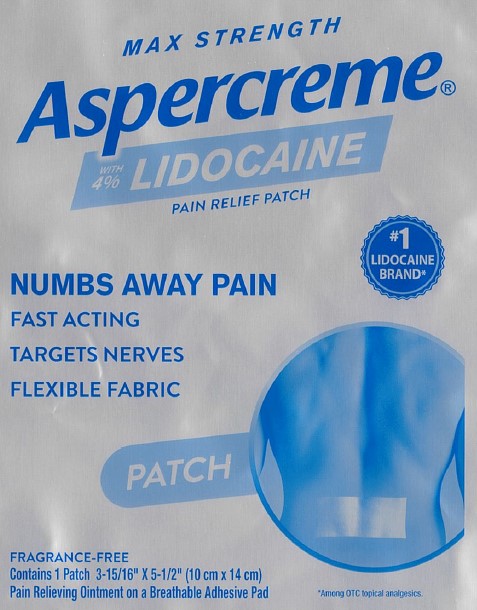
Package/Label Principal Display Panel – Lidocaine Patch US 1ct
MAX STRENGTH
Aspercreme®with 4% LIDOCAINE
PAIN RELIEF PATCH
NUMBS AWAY PAINFAST ACTING
TARGETS NERVES
FLEXIBLE FABRIC
#1 LIDOCAINE BRAND*
PATCH
FRAGRANCE-FREE
Contains 1 Patch 3-15/16” x 5-1/2” (10 cm x 14 cm)
Pain Relieving Ointment on a Breathable Adhesive Pad
*Among OTC topical analgesics.
Dist. by Chattem, Inc., a Sanofi Company
P.O. Box 2219, Chattanooga, TN
37409-0219 USA
©2022
www.aspercreme.com Made in Japan
Label
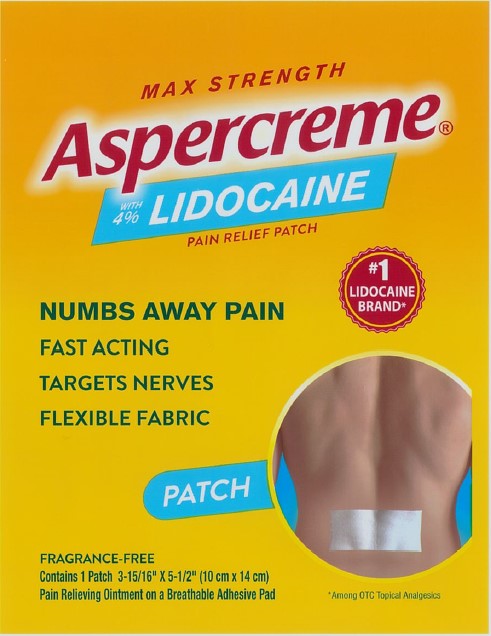
Package/Label Principal Display Panel – Lidocaine Patch Singles US 1ct
MAX STRENGTH
Aspercreme®with 4% LIDOCAINE
PAIN RELIEF PATCH
NUMBS AWAY PAINFAST ACTING
TARGETS NERVES
FLEXIBLE FABRIC
#1 LIDOCAINE BRAND*
PATCH
FRAGRANCE-FREE
Contains 1 Patch 3-15/16” x 5-1/2” (10 cm x 14 cm)
Pain Relieving Ointment on a Breathable Adhesive Pad
*Among OTC topical analgesics
CHATTEM®
A SANOFI COMPANY
Dist. by Chattem, Inc., a Sanofi Company
P.O. Box 2219, Chattanooga, TN
37409-0219 USA ©2020
www.aspercreme.com
Made in Japan
Label