Label: CAMILIA- matricaria recutita, rhubarb, phytolacca americana root liquid
-
NDC Code(s):
0220-9054-07,
0220-9054-08,
0220-9054-09,
0220-9054-10, view more0220-9054-46, 0220-9054-47
- Packager: Laboratoires Boiron
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
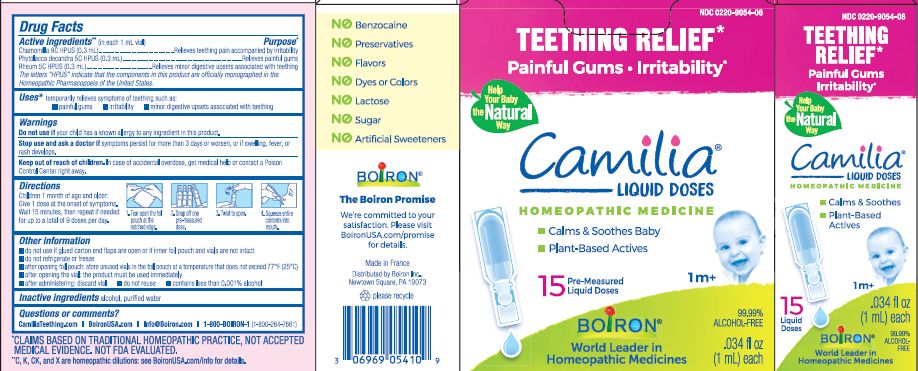
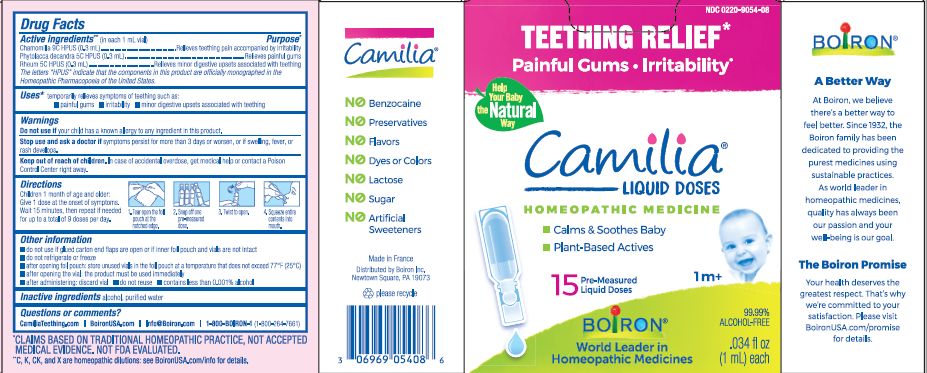
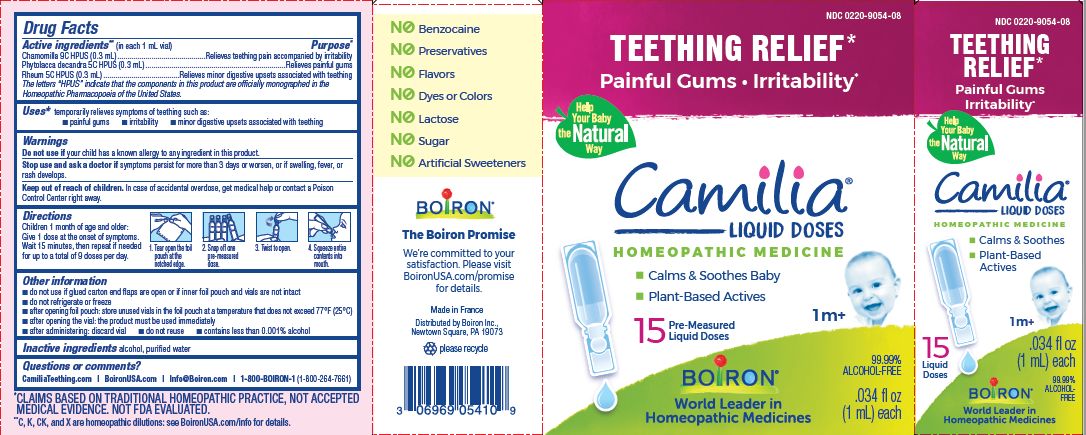
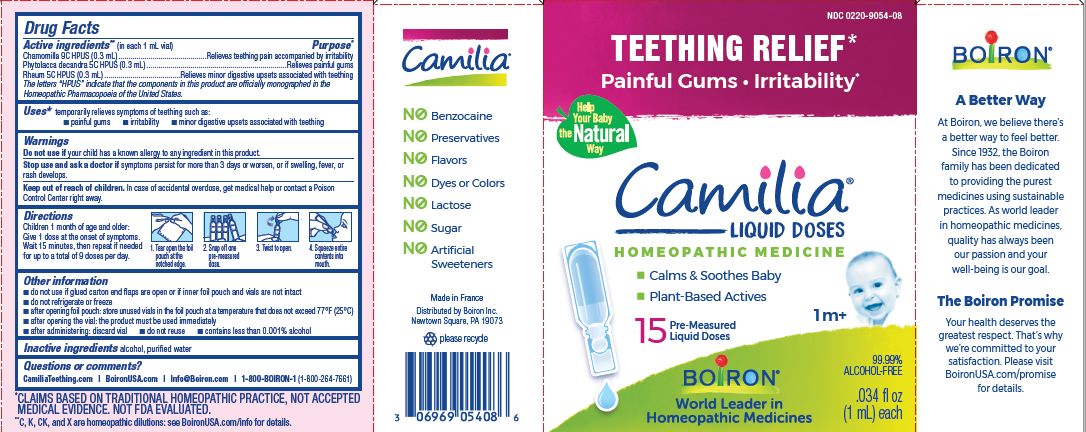
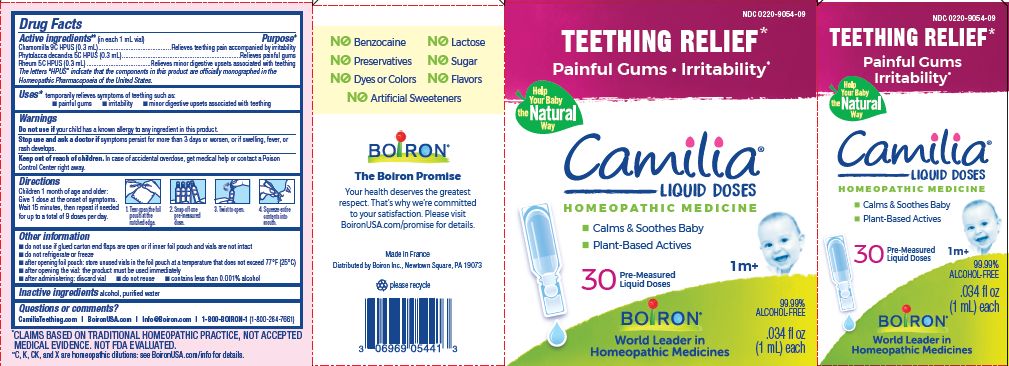
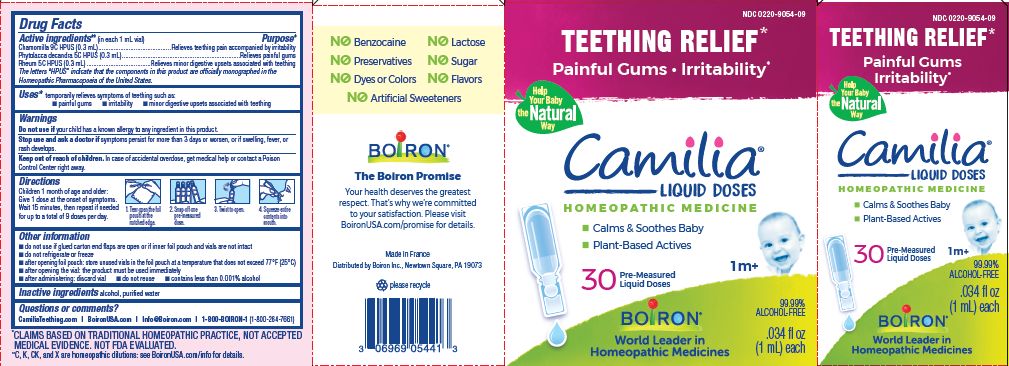
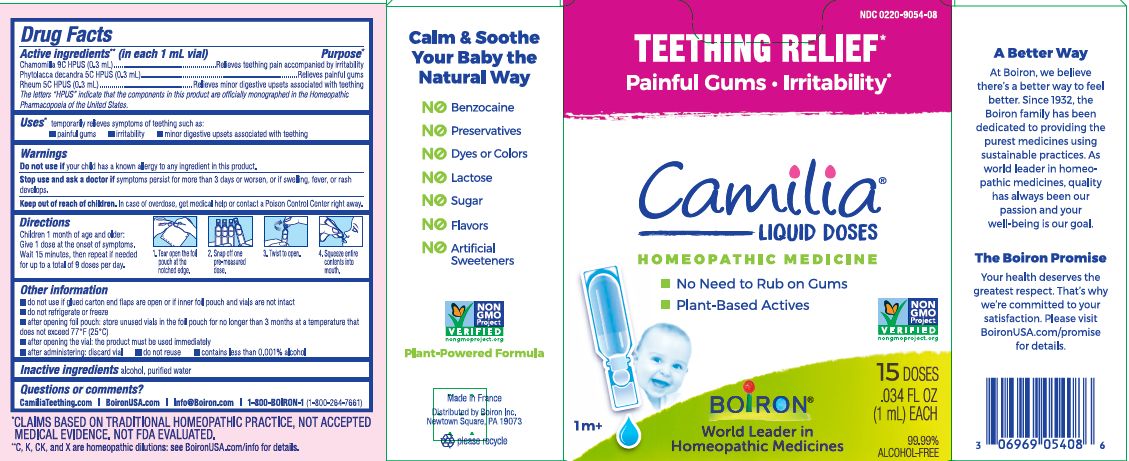
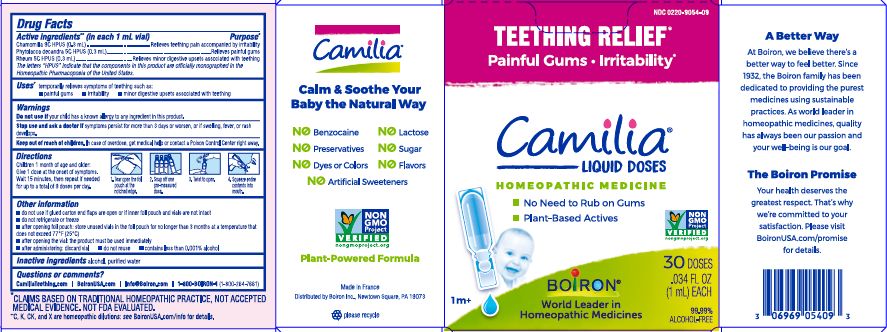
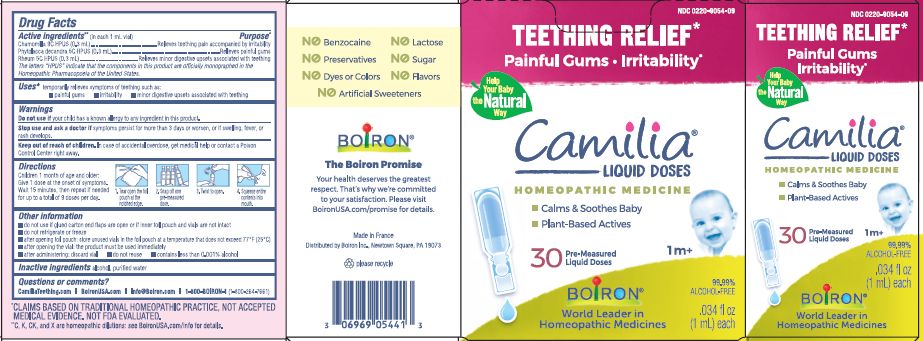
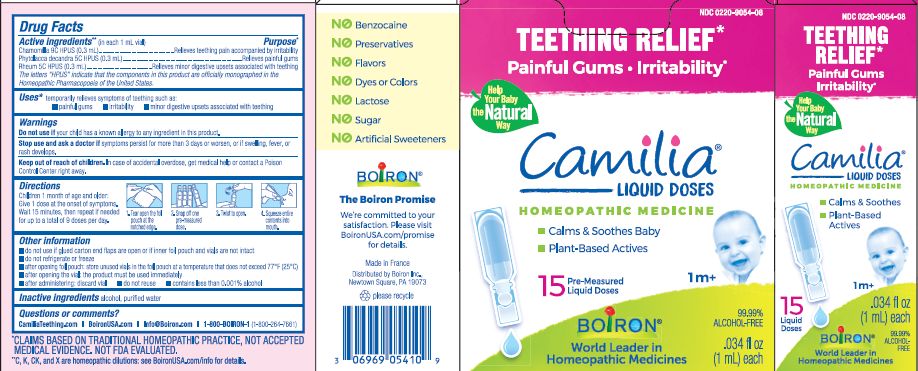
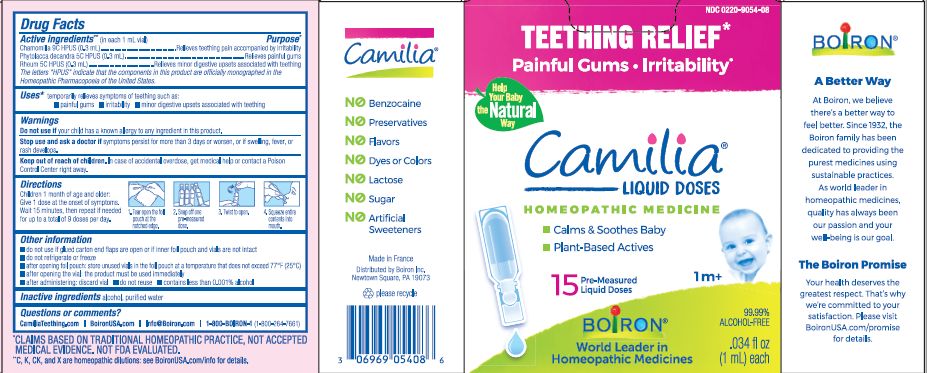
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
SPL UNCLASSIFIED SECTION
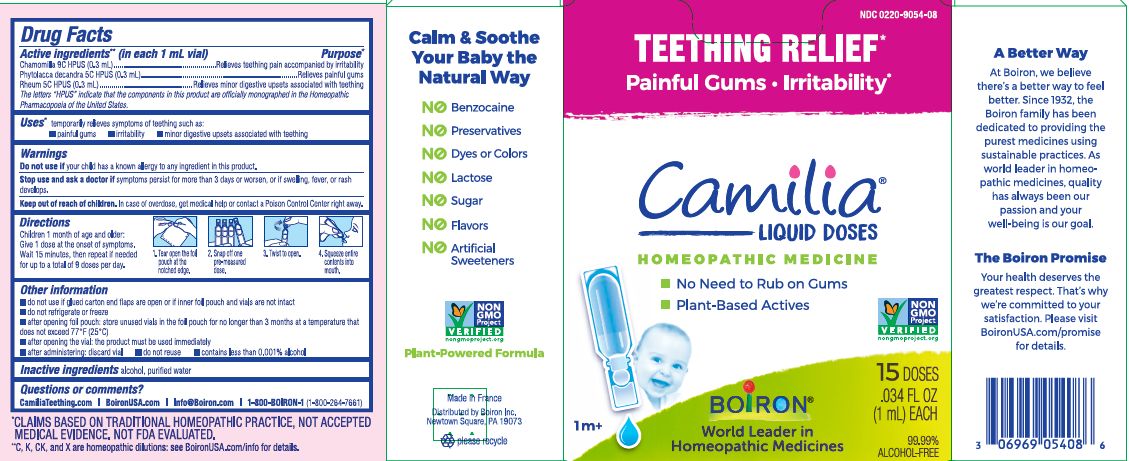
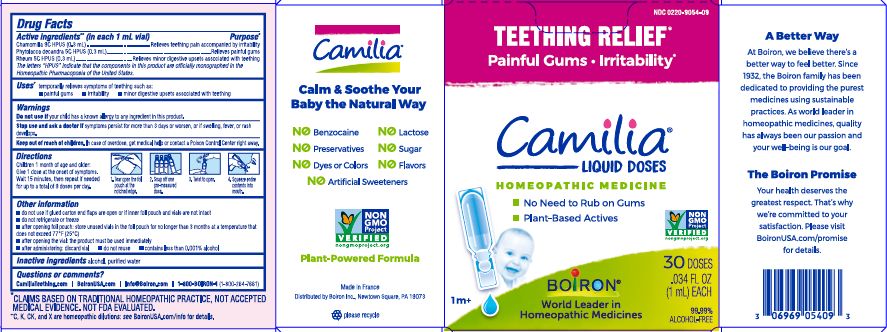
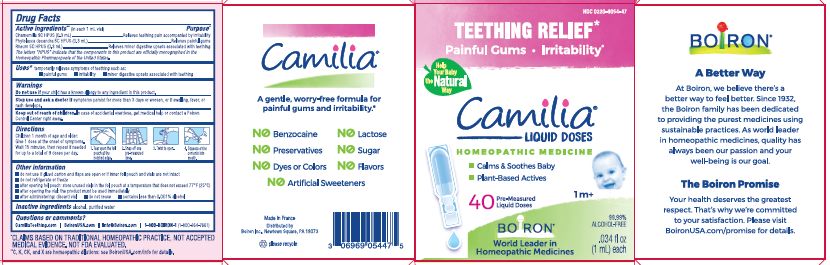
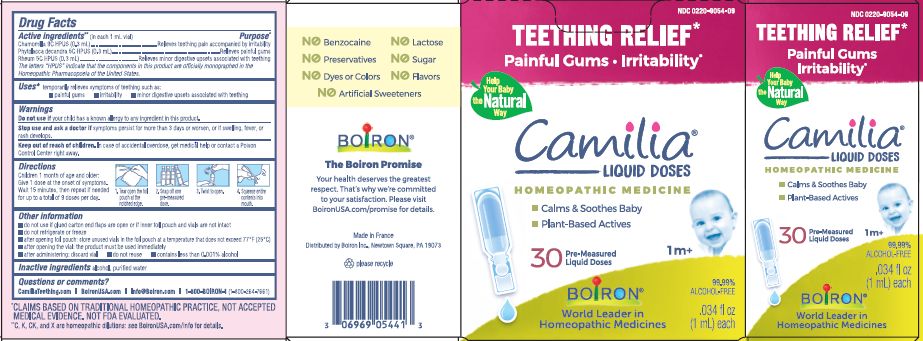
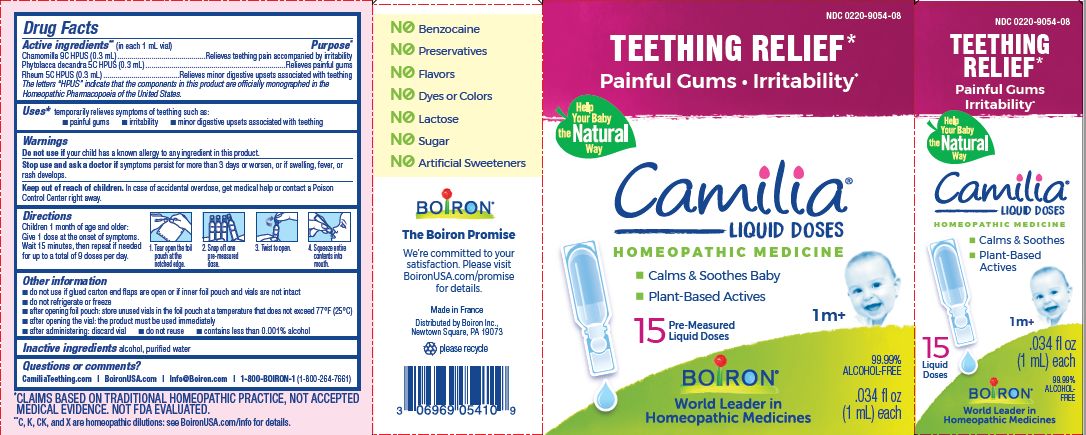
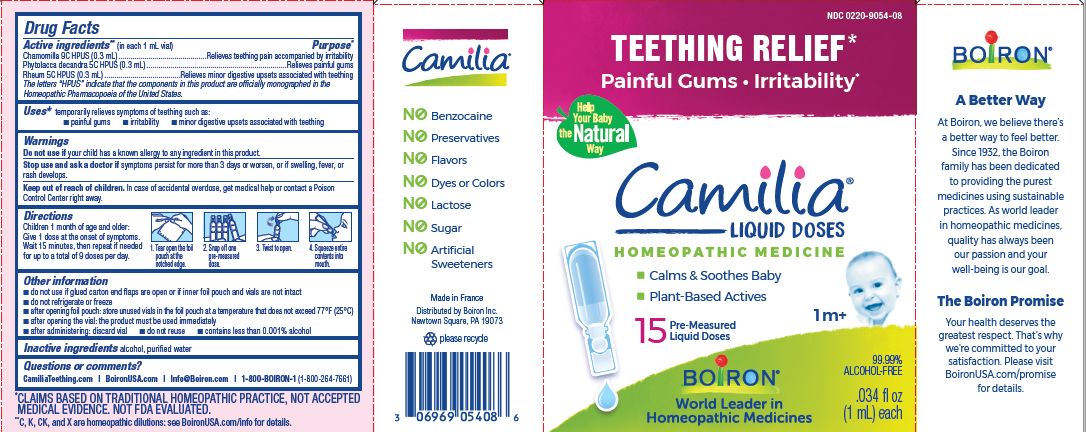
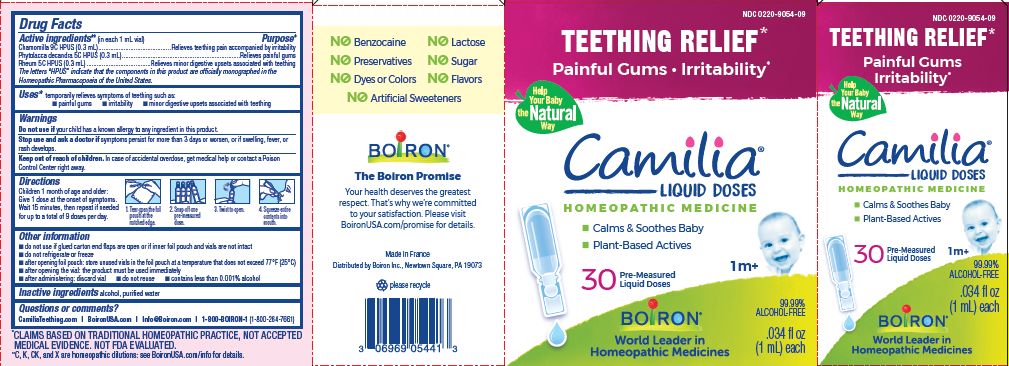
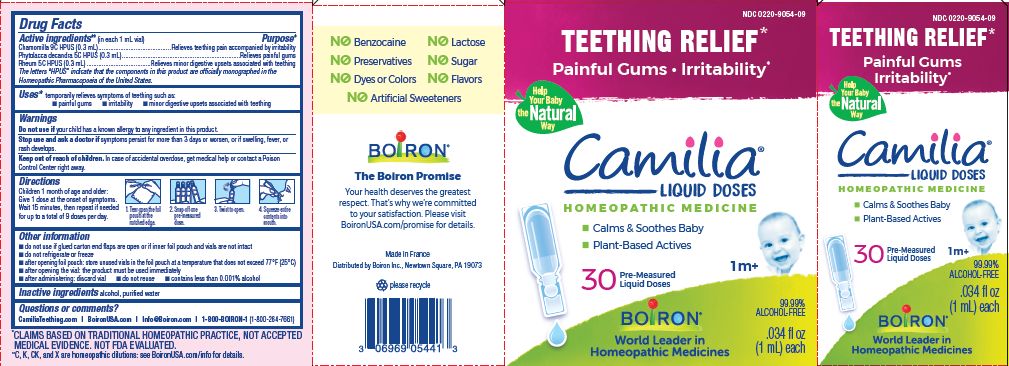
do not use if glued carton end flaps are open or if inner foil pouch and vials are not intact
do not refrigerate or freeze
after opening foil pouch: store unused vials in the foil pouch for no longer than 3 months at a temperature that does not exceed 77° F (25° C)
after opening the vial: the product must be used immediately
after administering: discard vial
do not reuse
contains less than 0.001% alcohol
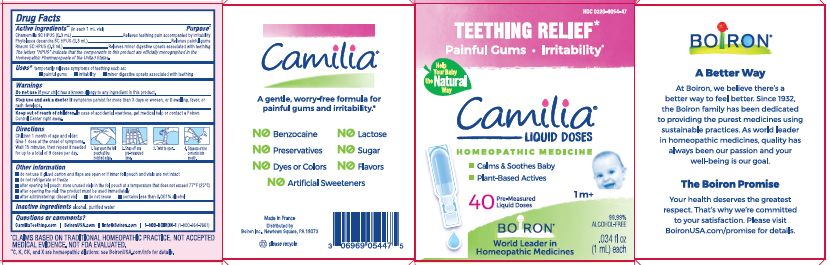
Teething Relief*
Painful Gums Irritability*
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details. - HOW SUPPLIED
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CAMILIA
matricaria recutita, rhubarb, phytolacca americana root liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9054 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 9 [hp_C] RHUBARB (UNII: G280W4MW6E) (RHUBARB - UNII:G280W4MW6E) RHUBARB 5 [hp_C] PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 5 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9054-46 1 in 1 PACKAGE 01/01/2014 1 10 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:0220-9054-07 1 in 1 PACKAGE 01/01/2014 2 5 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:0220-9054-10 150 in 1 CARTON 01/01/2014 02/27/2020 3 5 in 1 POUCH; Type 0: Not a Combination Product 4 NDC:0220-9054-47 1 in 1 PACKAGE 04/01/2020 4 40 in 1 POUCH; Type 0: Not a Combination Product 5 NDC:0220-9054-08 1 in 1 PACKAGE 12/01/2010 5 15 in 1 POUCH; Type 0: Not a Combination Product 6 NDC:0220-9054-09 1 in 1 PACKAGE 07/15/2011 6 30 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/01/2010 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 383674934 manufacture(0220-9054)