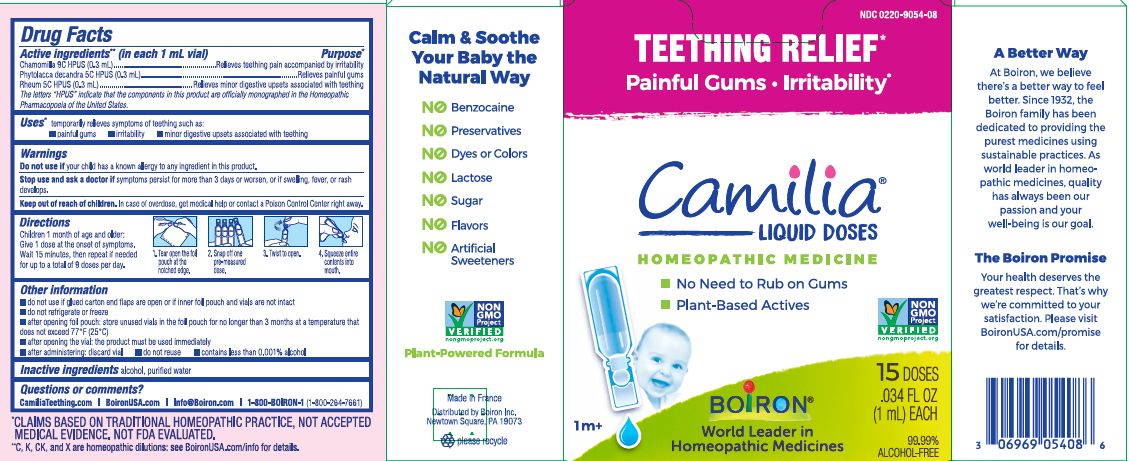
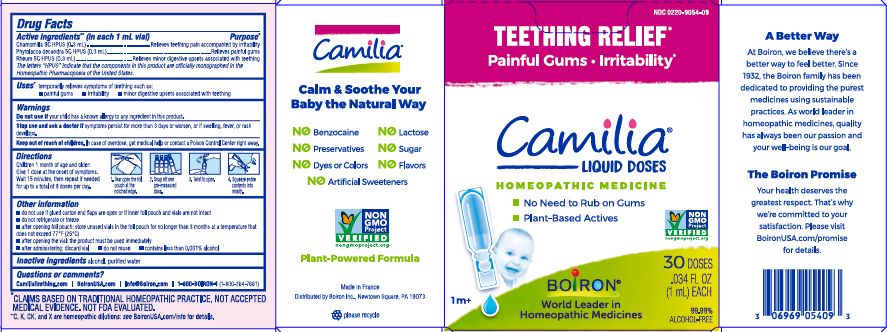
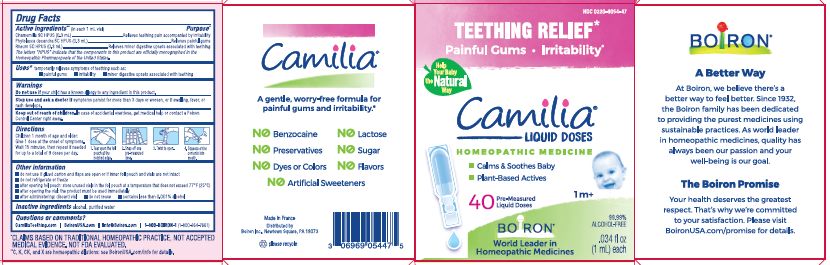
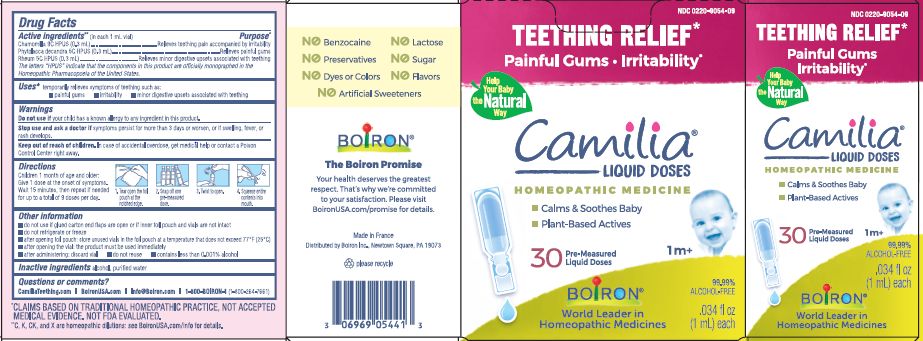
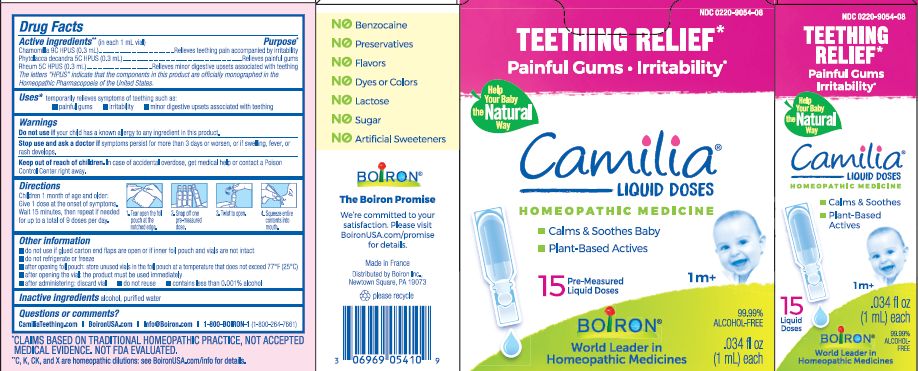
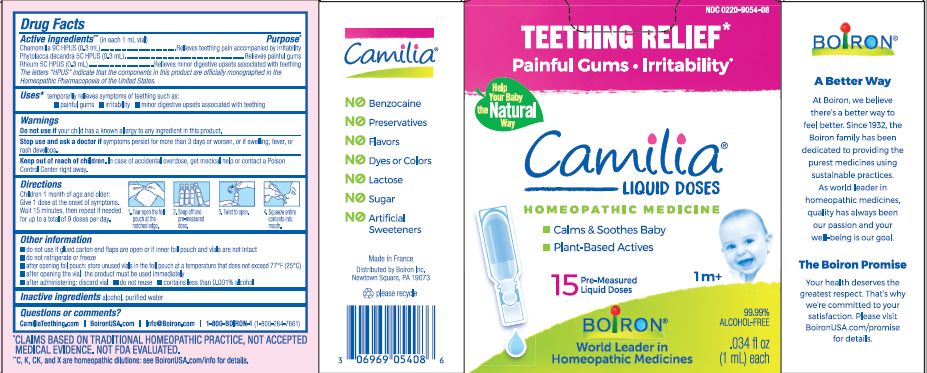
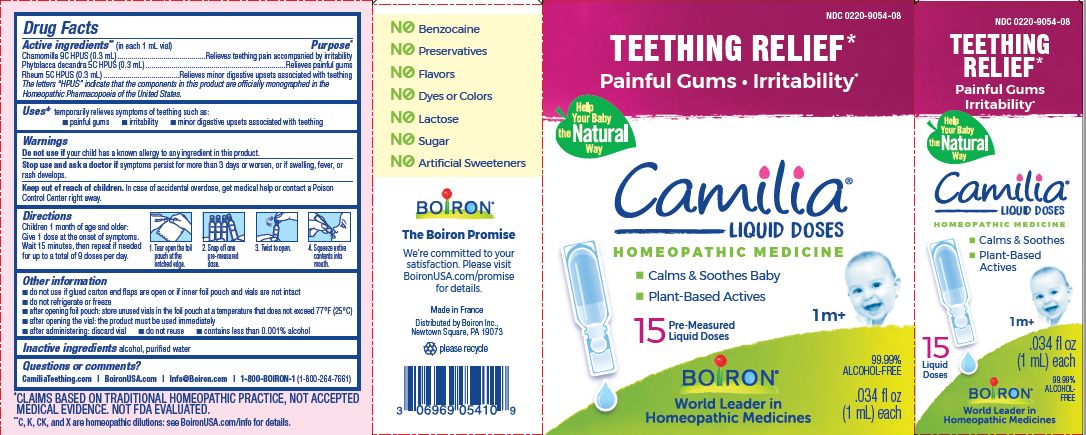
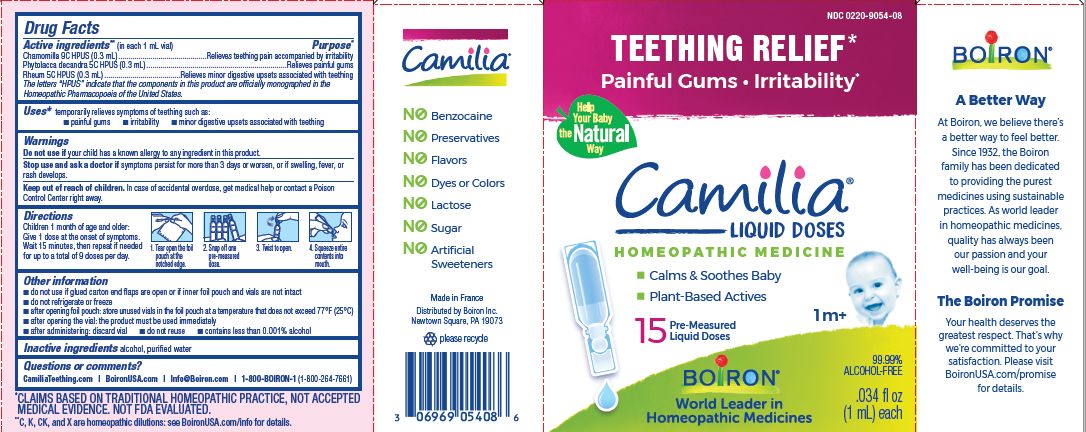
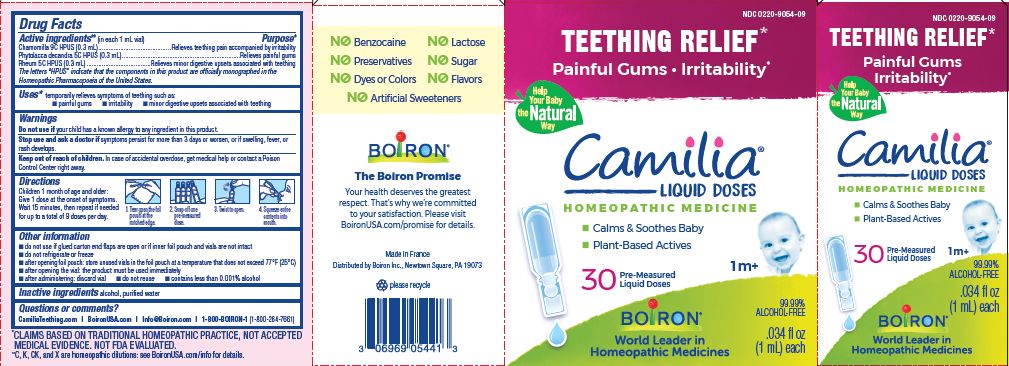
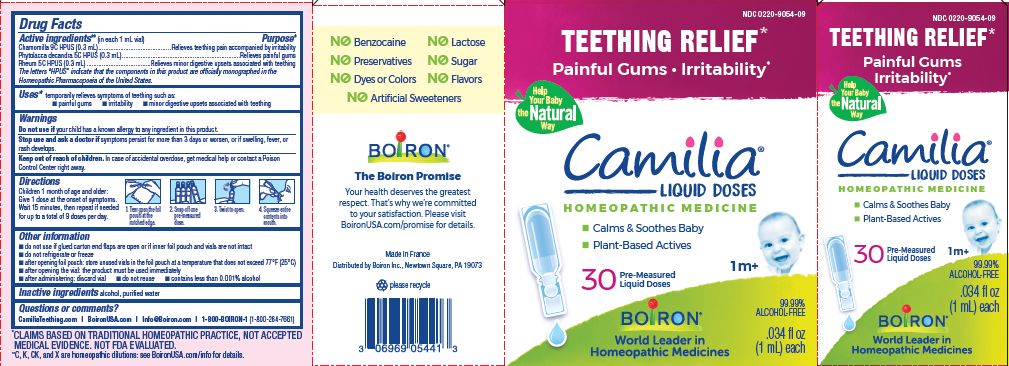
Active ingredients** (in each 1 mL vial)
Chamomilla 9C HPUS (0.3mL)
Phytolacca decandra 5C HPUS (0.3mL)
Rheum 5C HPUS (0.3mL)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Purpose*
Chamomilla 9C HPUS (0.3mL) ... Relieves teething pain accompanied by irritability
Phytolacca decandra 5C HPUS (0.3mL) ... Relieves painful gums
Rheum 5C HPUS (0.3mL) ... Relieves minor digestive disorders associated with teething
Uses*
temporarily relieves symptoms of teething such as:
- painful gums
- irritability
- minor digestive upsets associated with teething
Stop use and ask doctor if symptoms persist for more than 3 days or worsen, or if swelling, fever, or rash develops.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Children 1 month of age and older:
Give 1 dose at the onset of symptoms.
Wait 15 minutes, then repeat if needed for up to a total of 9 doses per day.
Tear open the foil pouch at the notched edge.
Snap off one pre-measured dose.
Twist to open.
Squeeze entire contents into mouth.
do not use if glued carton end flaps are open or if inner foil pouch and vials are not intact
do not refrigerate or freeze
after opening foil pouch: store unused vials in the foil pouch for no longer than 3 months at a temperature that does not exceed 77° F (25° C)
after opening the vial: the product must be used immediately
after administering: discard vial
do not reuse
contains less than 0.001% alcohol
Teething Relief*
Painful Gums Irritability*
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.