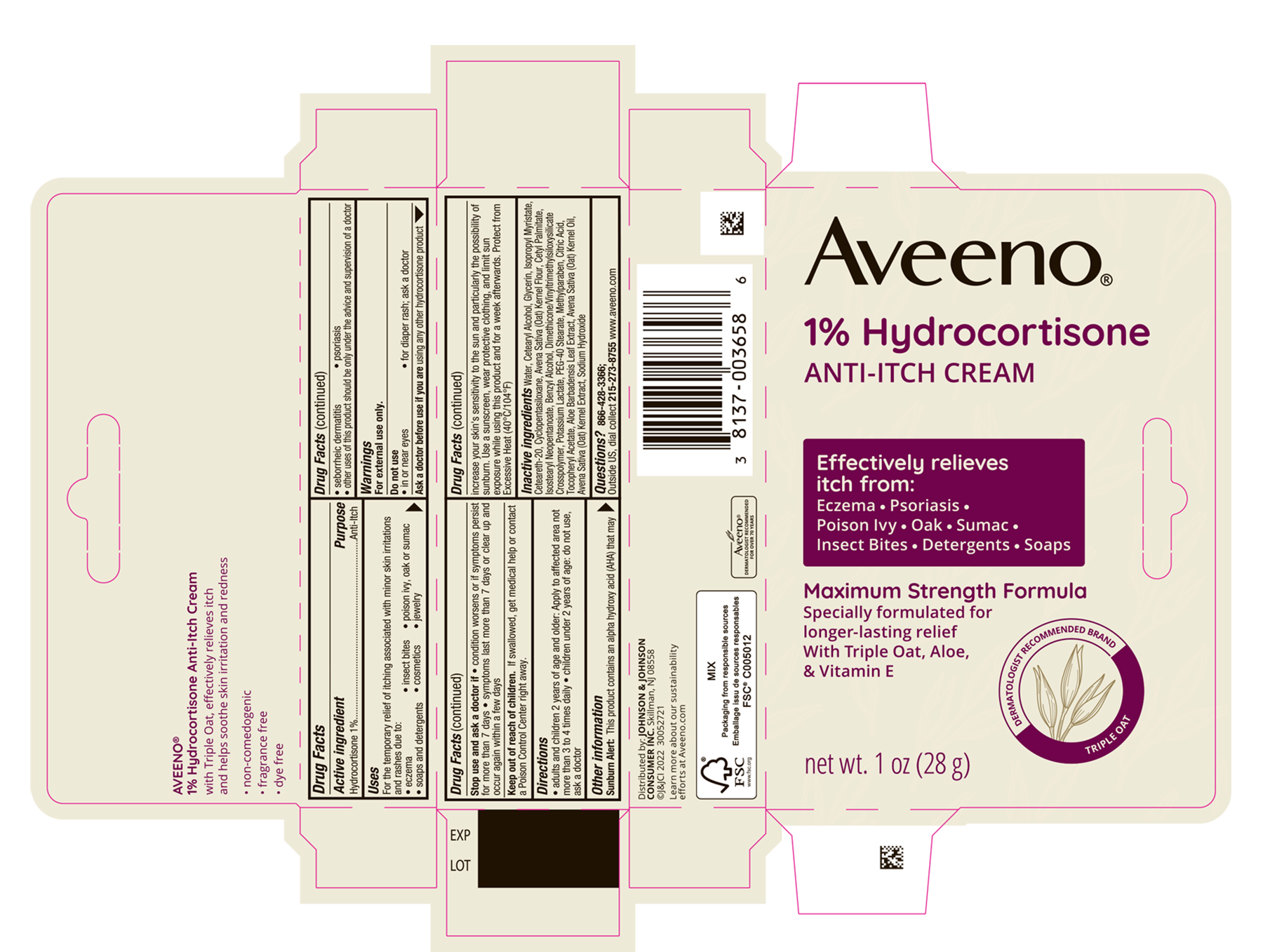
Label: AVEENO HYDROCORTISONE ANTI-ITCH- hydrocortisone cream
- NDC Code(s): 69968-0511-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Sunburn Alert
- Other information
-
Inactive ingredients
Water, Cetearyl Alcohol, Glycerin, Isopropyl Myristate, Cetereath-20, Cyclopentasiloxane, Avena Sativa (Oat) Kernel flour, Cetyl Palmitate, Isostearyl Neopentanoate, Benzyl Alcohol, Dimethicone/Vinyltrimethylsiloxysilicate Crosspolymer, Potassium Lactate, PEG-40 Stearate, Methylparaben, Citric Acid, Tocopheryl Acetate, Aloe Barbadensis Leaf Extract, Avena Sativa (Oat) Kernel Oil, Avena Sativa (Oat) Kernel Extract, Sodium Hydroxide
- Questions?
- SPL UNCLASSIFIED SECTION
-
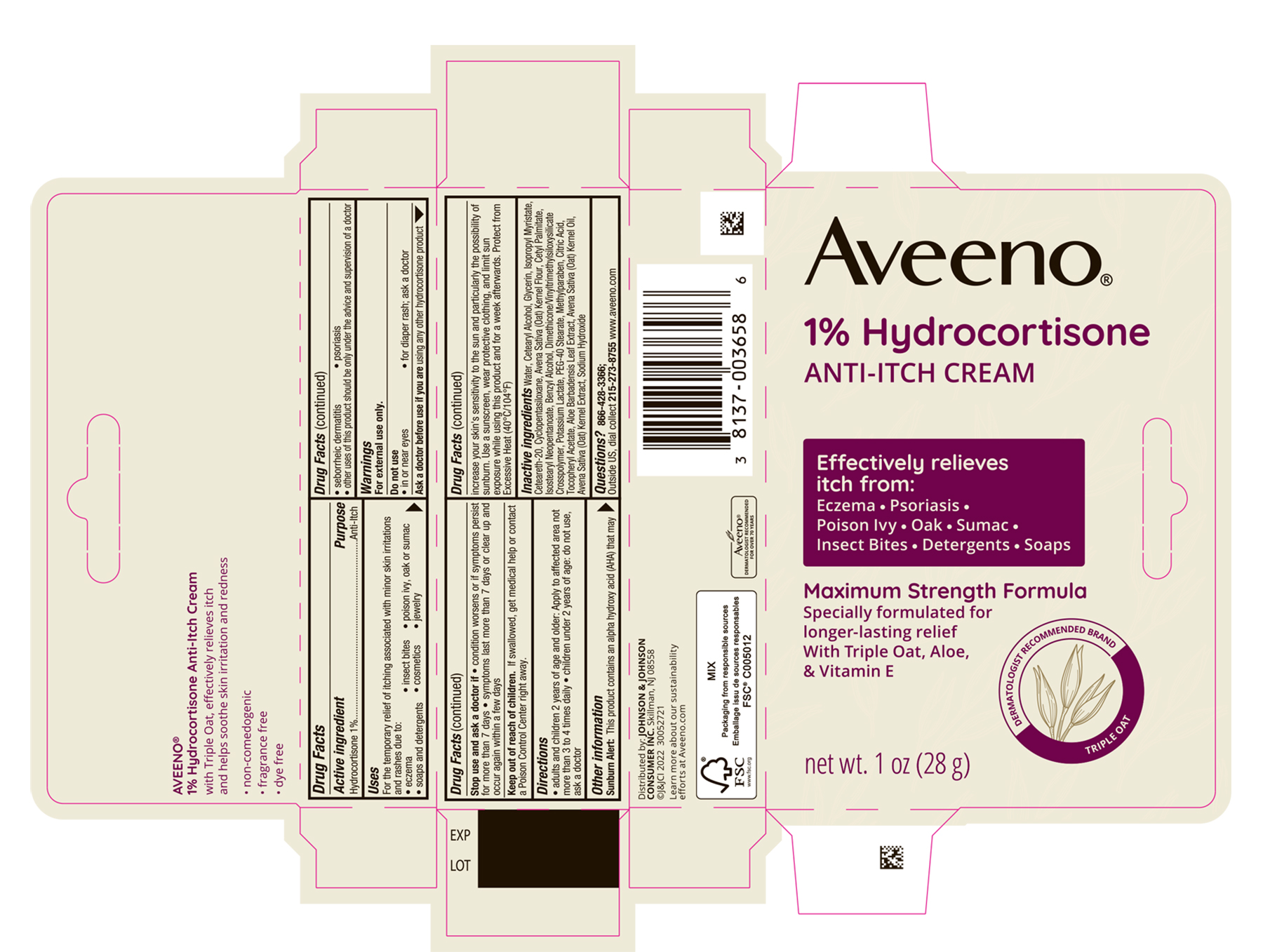
PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
Aveeno®
1% Hydrocortisone
ANTI-ITCH CREAM
Effectively relieves itch from:
Eczema ● Psoriasis
Poison Ivy ● Oak ● Sumac
Insect Bites ● Detergents ● Soaps
Maximum Strength Formula
Specially formulated for longer-lasting relief
With Triple Oat, Aloe, & Vitamin E
DERMATOLOGIST RECOMMENDED BRAND
TRIPLE OAT
net wt. 1 oz (28 g)
-
INGREDIENTS AND APPEARANCE
AVEENO HYDROCORTISONE ANTI-ITCH
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0511 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OATMEAL (UNII: 8PI54V663Y) CETYL PALMITATE (UNII: 5ZA2S6B08X) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) BENZYL ALCOHOL (UNII: LKG8494WBH) POTASSIUM LACTATE (UNII: 87V1KMK4QV) METHYLPARABEN (UNII: A2I8C7HI9T) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OAT KERNEL OIL (UNII: 3UVP41R77R) OAT (UNII: Z6J799EAJK) ALOE VERA LEAF (UNII: ZY81Z83H0X) SODIUM HYDROXIDE (UNII: 55X04QC32I) PEG-40 STEARATE (UNII: ECU18C66Q7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0511-1 1 in 1 CARTON 03/01/2019 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/01/2019 Labeler - Johnson & Johnson Consumer Inc. (118772437)