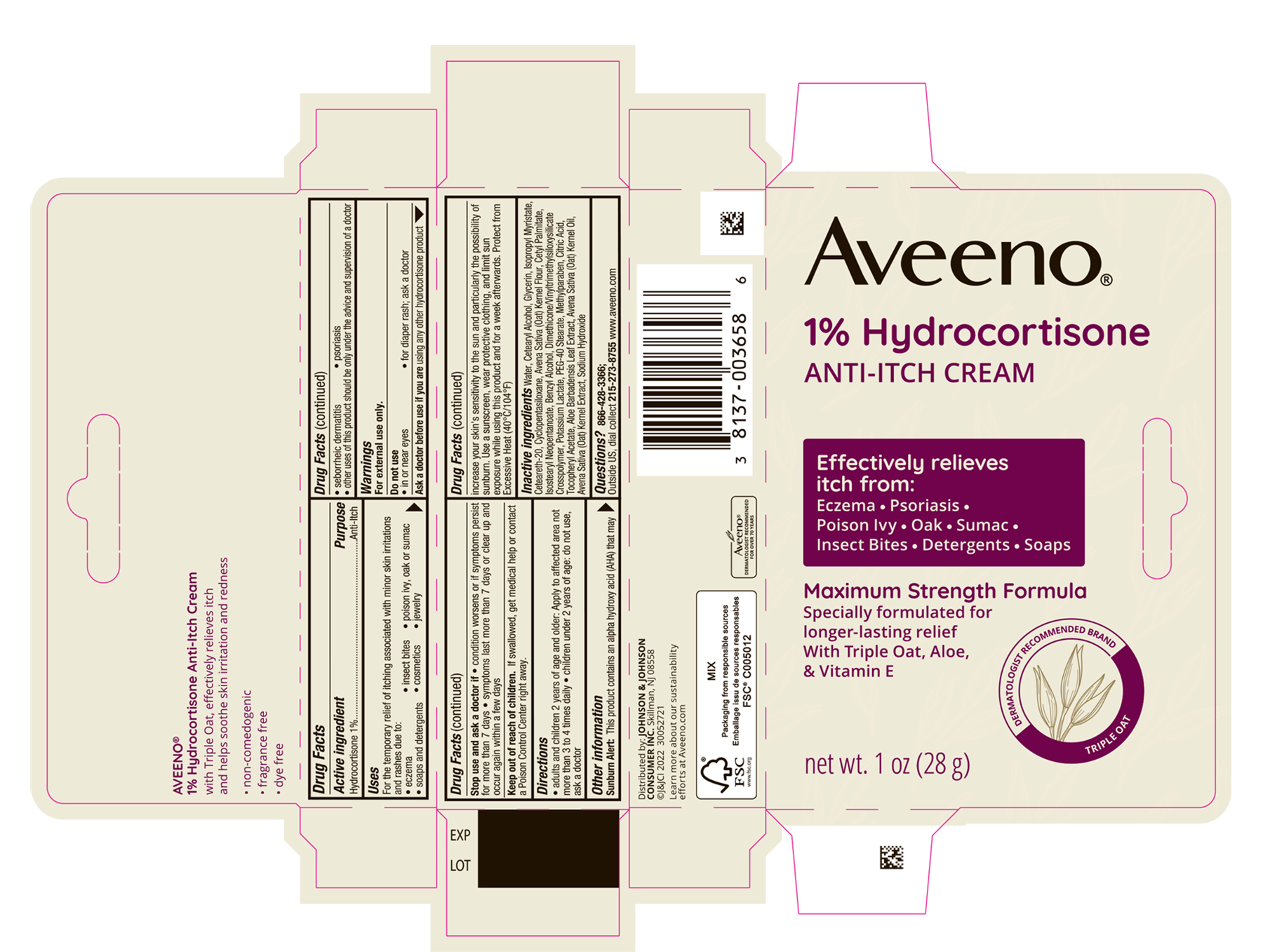
Use
For the temporary relief of itching associated with minor skin irritations and rashes due to:
- eczema
- insect bites
- poison ivy, oak or sumac
- soaps and detergents
- cosmetics
- jewelry
- seborrheic dermatitis
- psoriasis
- Other uses of this product should be only under the advice and supervision of doctor
Warnings
For external use only.
Directions
- Adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age, do not use; ask a doctor.
Sunburn Alert
This product contains an alpha hydroxy acid (AHA) that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product and for a week afterwards.
Inactive ingredients
Water, Cetearyl Alcohol, Glycerin, Isopropyl Myristate, Cetereath-20, Cyclopentasiloxane, Avena Sativa (Oat) Kernel flour, Cetyl Palmitate, Isostearyl Neopentanoate, Benzyl Alcohol, Dimethicone/Vinyltrimethylsiloxysilicate Crosspolymer, Potassium Lactate, PEG-40 Stearate, Methylparaben, Citric Acid, Tocopheryl Acetate, Aloe Barbadensis Leaf Extract, Avena Sativa (Oat) Kernel Oil, Avena Sativa (Oat) Kernel Extract, Sodium Hydroxide
PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
Aveeno®
1% Hydrocortisone
ANTI-ITCH CREAM
Effectively relieves itch from:
Eczema ● Psoriasis
Poison Ivy ● Oak ● Sumac
Insect Bites ● Detergents ● Soaps
Maximum Strength Formula
Specially formulated for longer-lasting relief
With Triple Oat, Aloe, & Vitamin E
DERMATOLOGIST RECOMMENDED BRAND
TRIPLE OAT
net wt. 1 oz (28 g)