Label: SEBOBALANCE PURIFYING SEBO-ABSORBING MASK- salicylic acid paste
-
Contains inactivated NDC Code(s)
NDC Code(s): 62499-535-08, 62499-535-09, 62499-535-11 - Packager: Laboratoire Dr. Renaud
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 29, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
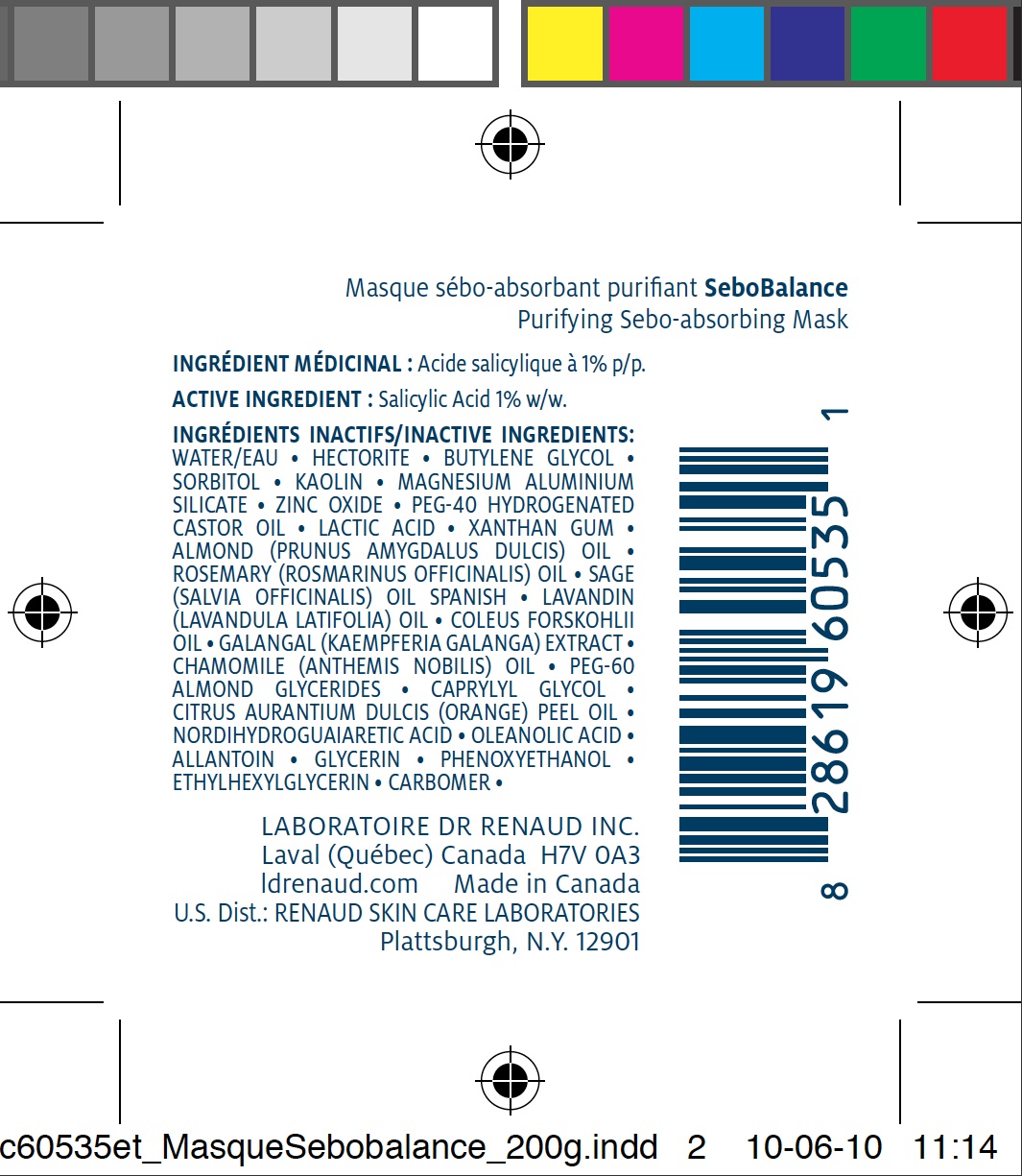
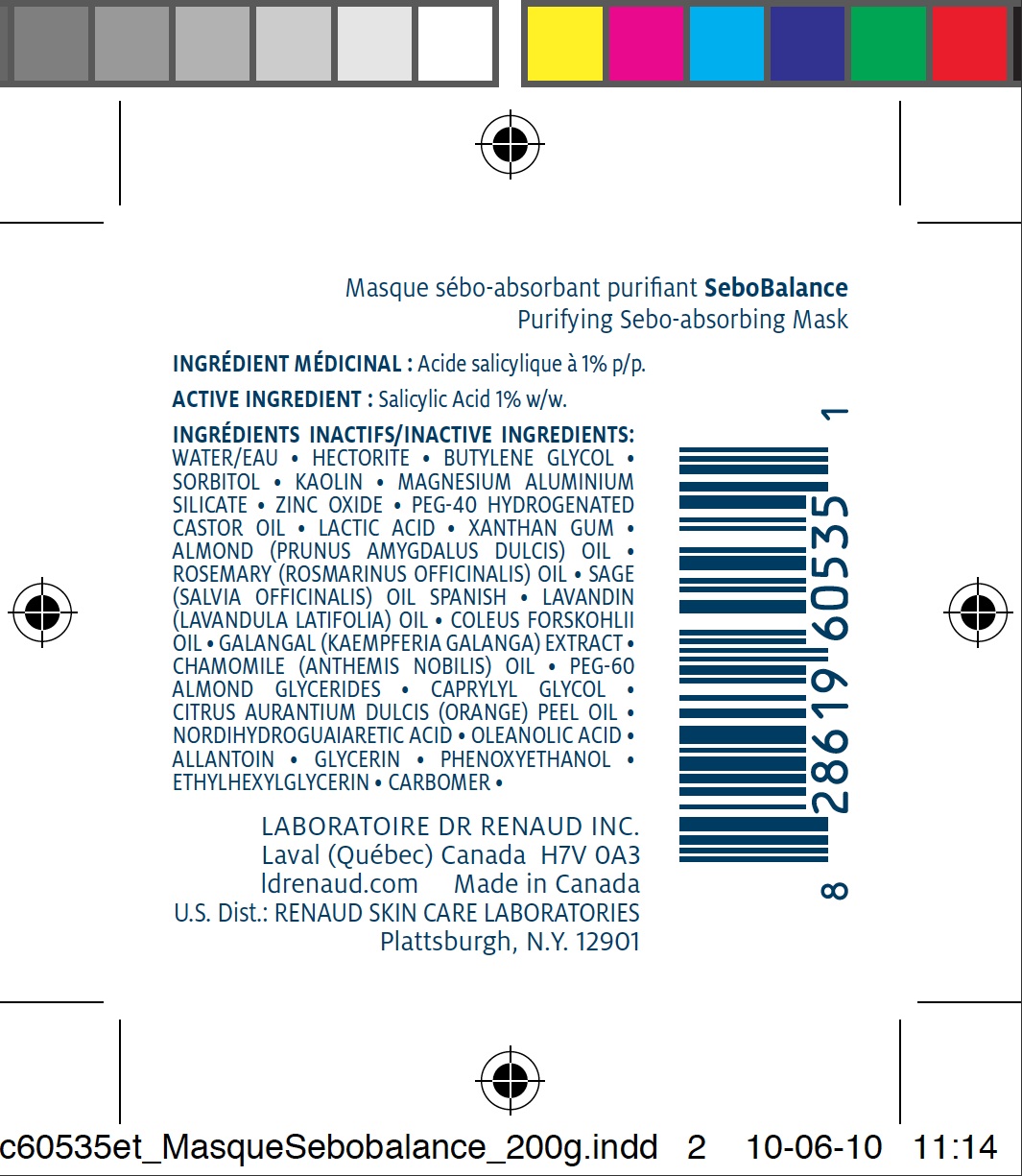
ACTIVE INGREDIENT
Salicylic Acid 1% w/w.
INACTIVE INGREDIENTS
WATER/EAU • HECTORITE • BUTYLENE GLYCOL • SORBITOL • KAOLIN • MAGNESIUM ALUMINIUM SILICATE • ZINC OXIDE • PEG-40 HYDROGENATED CASTOR OIL • LACTIC ACID • XANTHAN GUM • ALMOND (PRUNUS AMYGDALUS DULCIS) OIL • ROSEMARY (ROSMARINUS OFFICINALIS) OIL • SAGE (SALVIA OFFICINALIS) OIL SPANISH • LAVANDIN (LAVANDULA LATIFOLIA) OIL • COLEUS FORSKOHLII OIL • GALANGAL (KAEMPFERIA GALANGA) EXTRACT • CHAMOMILE (ANTHEMIS NOBILIS) OIL • PEG-60 ALMOND GLYCERIDES • CAPRYLYL GLYCOL • CITRUS AURANTIUM DULCIS (ORANGE) PEEL OIL • NORDIHYDROGUAIARETIC ACID • OLEANOLIC ACID • ALLANTOIN • GLYCERIN • PHENOXYETHANOL • ETHYLHEXYLGLYCERIN • CARBOMER •DIRECTIONS
Once or twice a week, after
a thorough cleansing of the skin, apply evenly
and sparingly to the face and the neck.
Pause for 20 minutes. Rinse liberally first with
lukewarm water, then with cold.WARNINGS
For external use only.
Using other topical acne medications at the same
time or immediately following use of this product
may increase dryness or irritation of the skin.
If this occurs, only one medication should be
used unless directed by a doctor. Avoid
direct contact with the eyes. If product gets
into the eyes, rinse liberally with water.
Discontinue use if skin irritation develops or
increases. If irritation persists, consult a doctor. - PRINCIPAL DISPLAY PANEL
- KEEP OUT OF REACH OF CHILDREN
-
INGREDIENTS AND APPEARANCE
SEBOBALANCE PURIFYING SEBO-ABSORBING MASK
salicylic acid pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62499-535 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HECTORITE (UNII: 08X4KI73EZ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SORBITOL (UNII: 506T60A25R) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) ZINC OXIDE (UNII: SOI2LOH54Z) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) LACTIC ACID (UNII: 33X04XA5AT) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALMOND OIL (UNII: 66YXD4DKO9) ROSEMARY OIL (UNII: 8LGU7VM393) SAGE OIL (UNII: U27K0H1H2O) LAVANDIN OIL (UNII: 9RES347CKG) GALANGAL OIL (UNII: K0951TAR6Z) CHAMOMILE FLOWER OIL (UNII: 60F80Z61A9) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ORANGE OIL (UNII: AKN3KSD11B) ALLANTOIN (UNII: 344S277G0Z) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CARBOMER 934 (UNII: Z135WT9208) MASOPROCOL (UNII: 7BO8G1BYQU) OLEANOLIC ACID (UNII: 6SMK8R7TGJ) Product Characteristics Color blue (dark bleu) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62499-535-11 1 in 1 CARTON 1 NDC:62499-535-08 60 g in 1 TUBE 2 NDC:62499-535-09 200 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 06/29/2010 Labeler - Laboratoire Dr. Renaud (202501565)