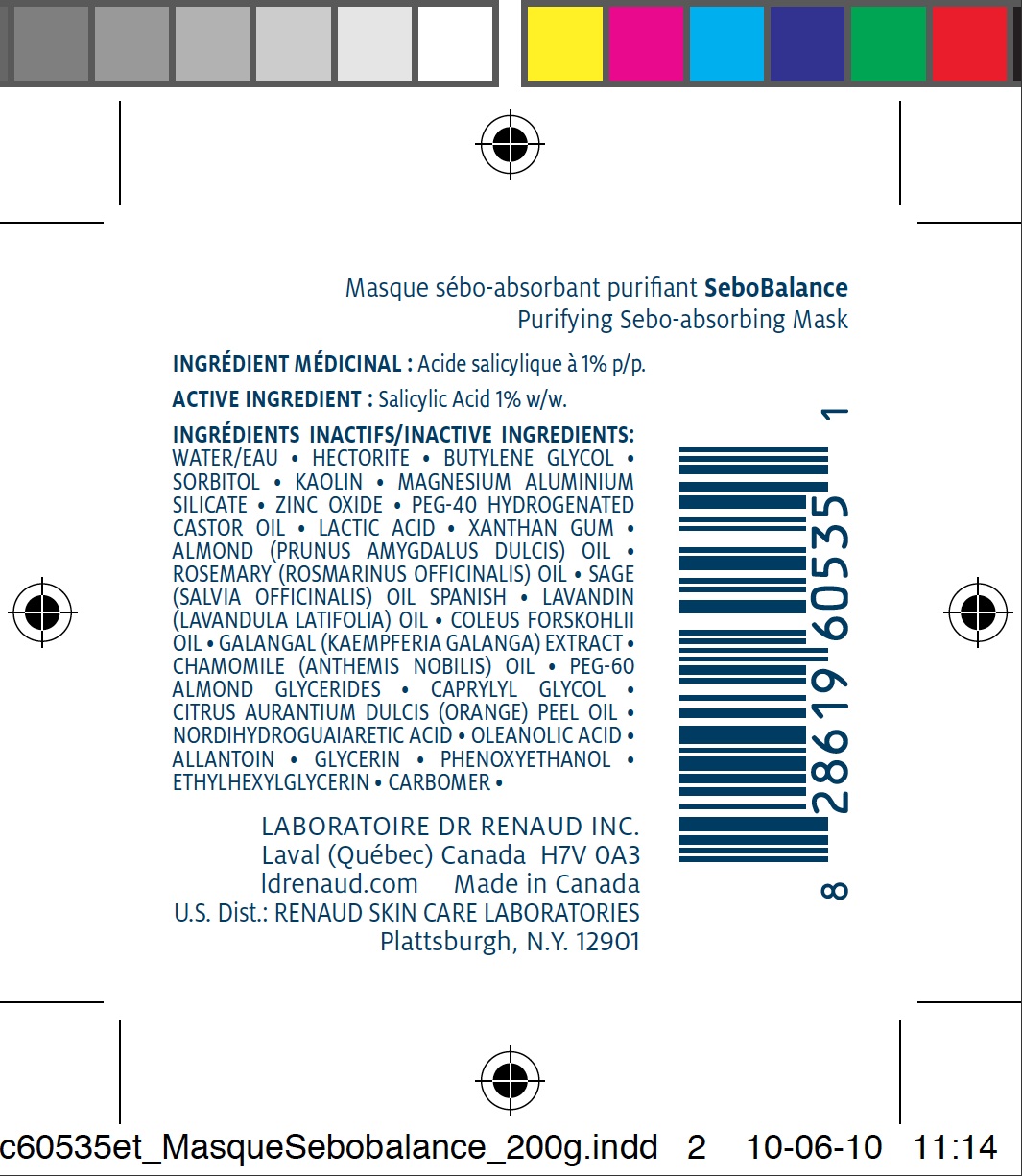
ACTIVE INGREDIENT
Salicylic Acid 1% w/w.INACTIVE INGREDIENTS
WATER/EAU • HECTORITE • BUTYLENE GLYCOL • SORBITOL • KAOLIN • MAGNESIUM ALUMINIUM SILICATE • ZINC OXIDE • PEG-40 HYDROGENATED CASTOR OIL • LACTIC ACID • XANTHAN GUM • ALMOND (PRUNUS AMYGDALUS DULCIS) OIL • ROSEMARY (ROSMARINUS OFFICINALIS) OIL • SAGE (SALVIA OFFICINALIS) OIL SPANISH • LAVANDIN (LAVANDULA LATIFOLIA) OIL • COLEUS FORSKOHLII OIL • GALANGAL (KAEMPFERIA GALANGA) EXTRACT • CHAMOMILE (ANTHEMIS NOBILIS) OIL • PEG-60 ALMOND GLYCERIDES • CAPRYLYL GLYCOL • CITRUS AURANTIUM DULCIS (ORANGE) PEEL OIL • NORDIHYDROGUAIARETIC ACID • OLEANOLIC ACID • ALLANTOIN • GLYCERIN • PHENOXYETHANOL • ETHYLHEXYLGLYCERIN • CARBOMER •DIRECTIONS
Once or twice a week, aftera thorough cleansing of the skin, apply evenly
and sparingly to the face and the neck.
Pause for 20 minutes. Rinse liberally first with
lukewarm water, then with cold.
WARNINGS
For external use only.Using other topical acne medications at the same
time or immediately following use of this product
may increase dryness or irritation of the skin.
If this occurs, only one medication should be
used unless directed by a doctor. Avoid
direct contact with the eyes. If product gets
into the eyes, rinse liberally with water.
Discontinue use if skin irritation develops or
increases. If irritation persists, consult a doctor.