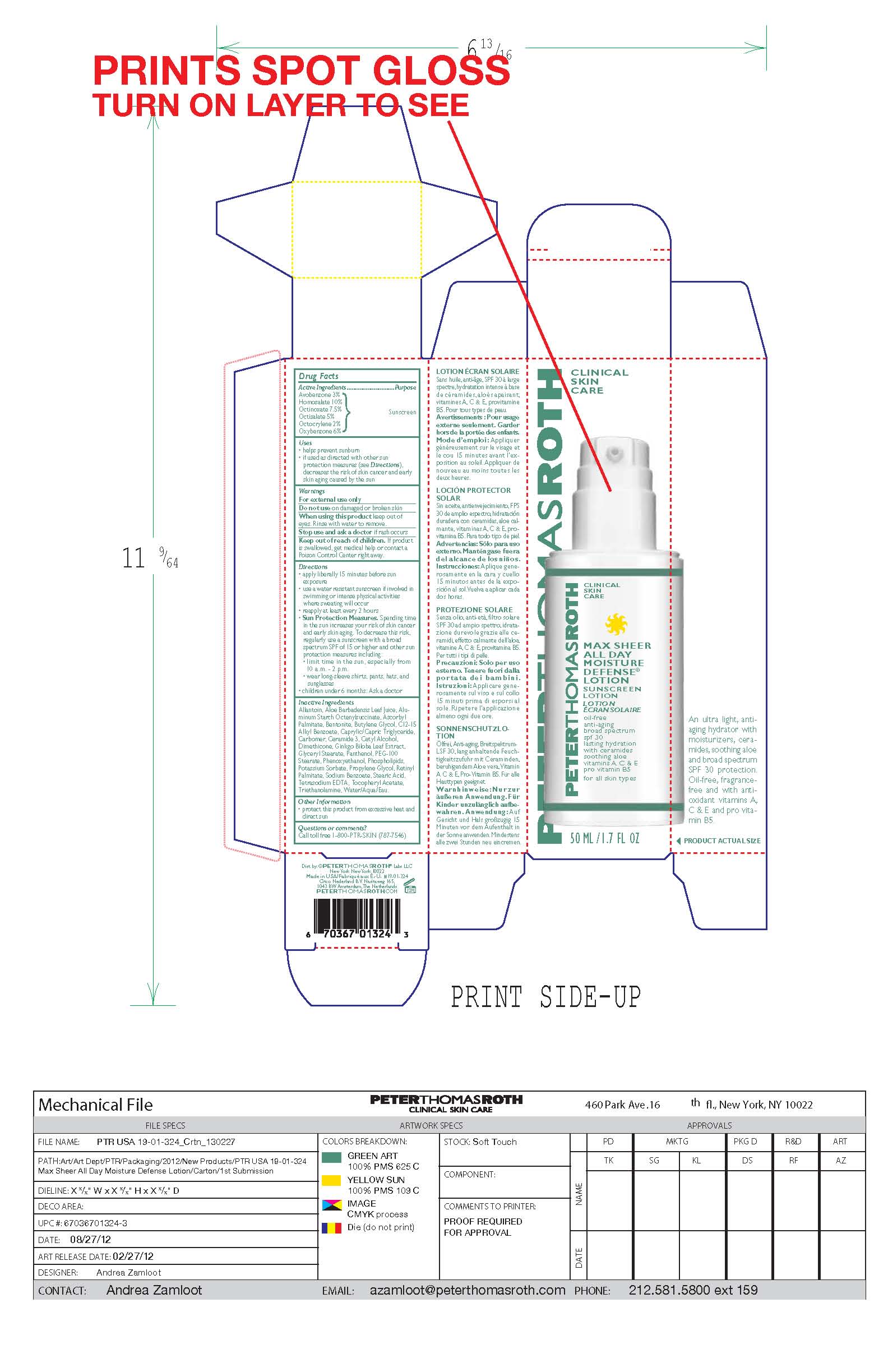
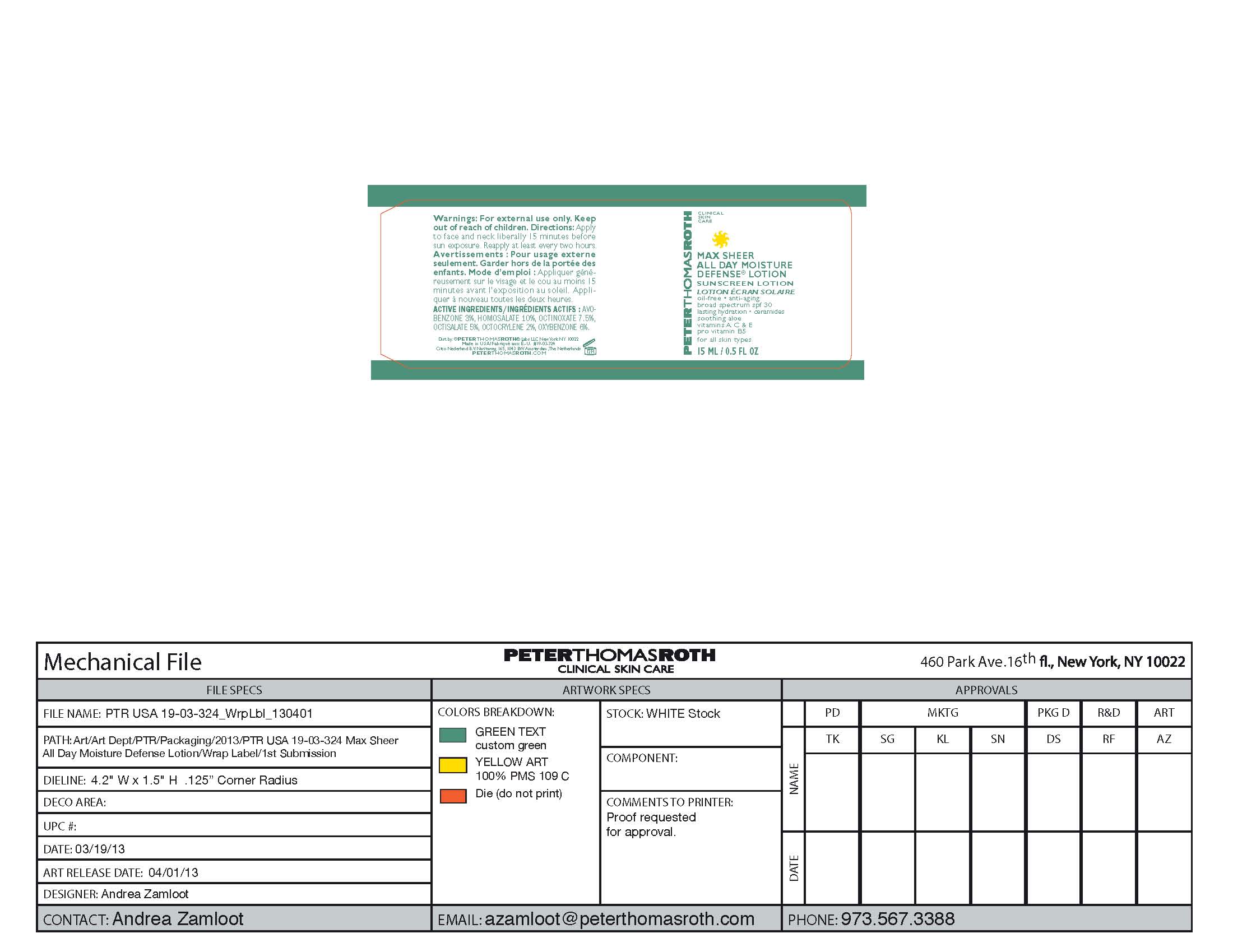
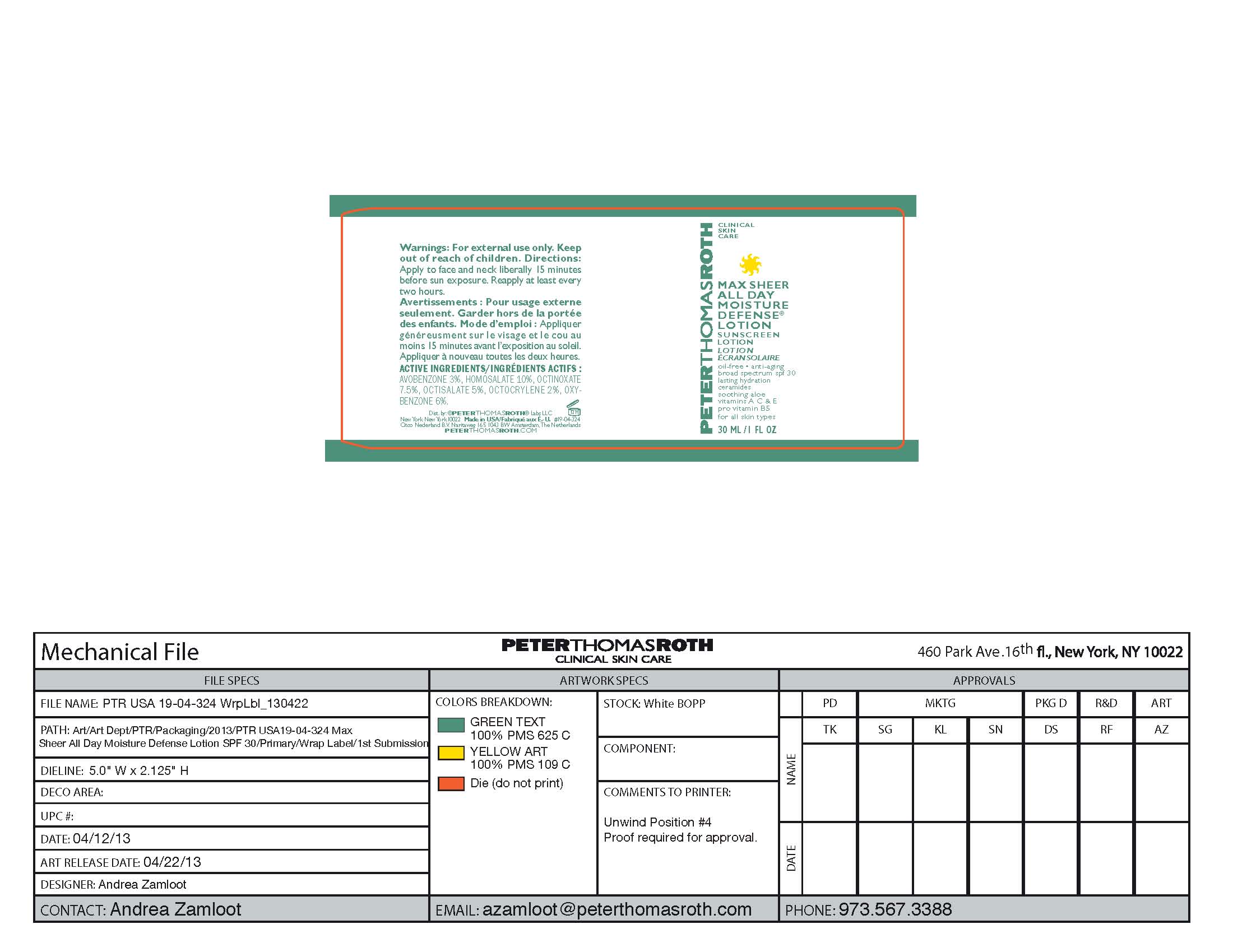
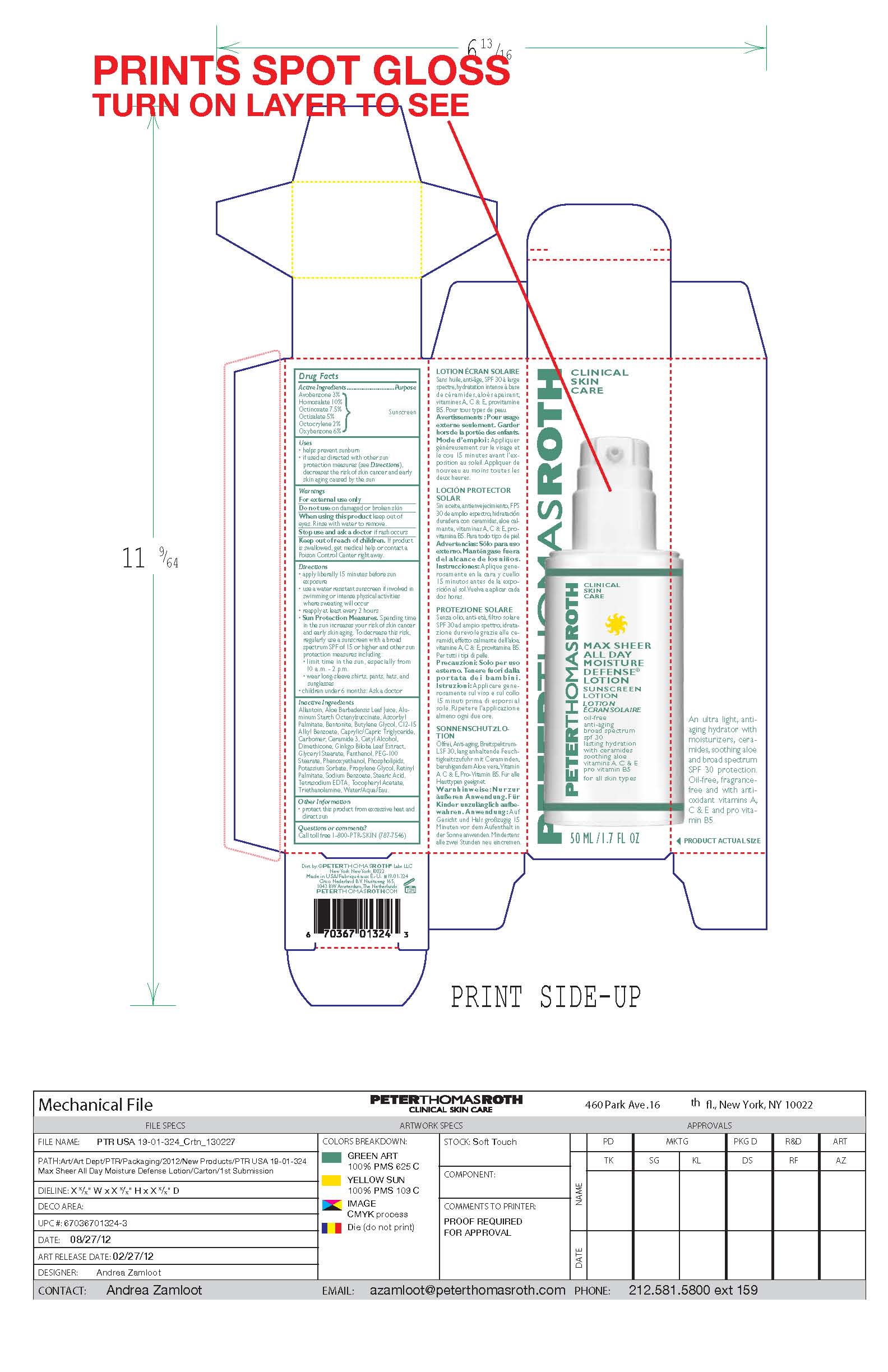
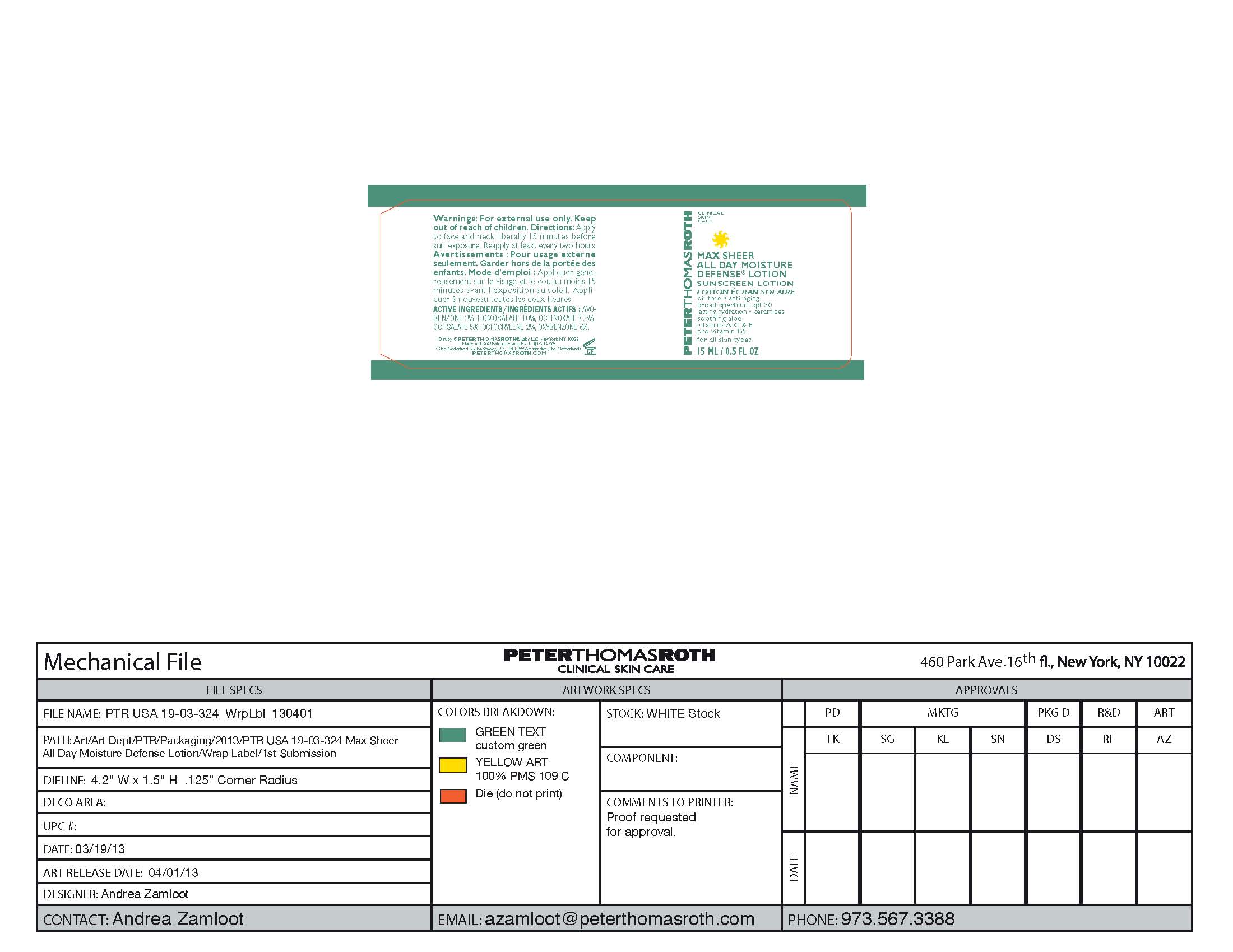
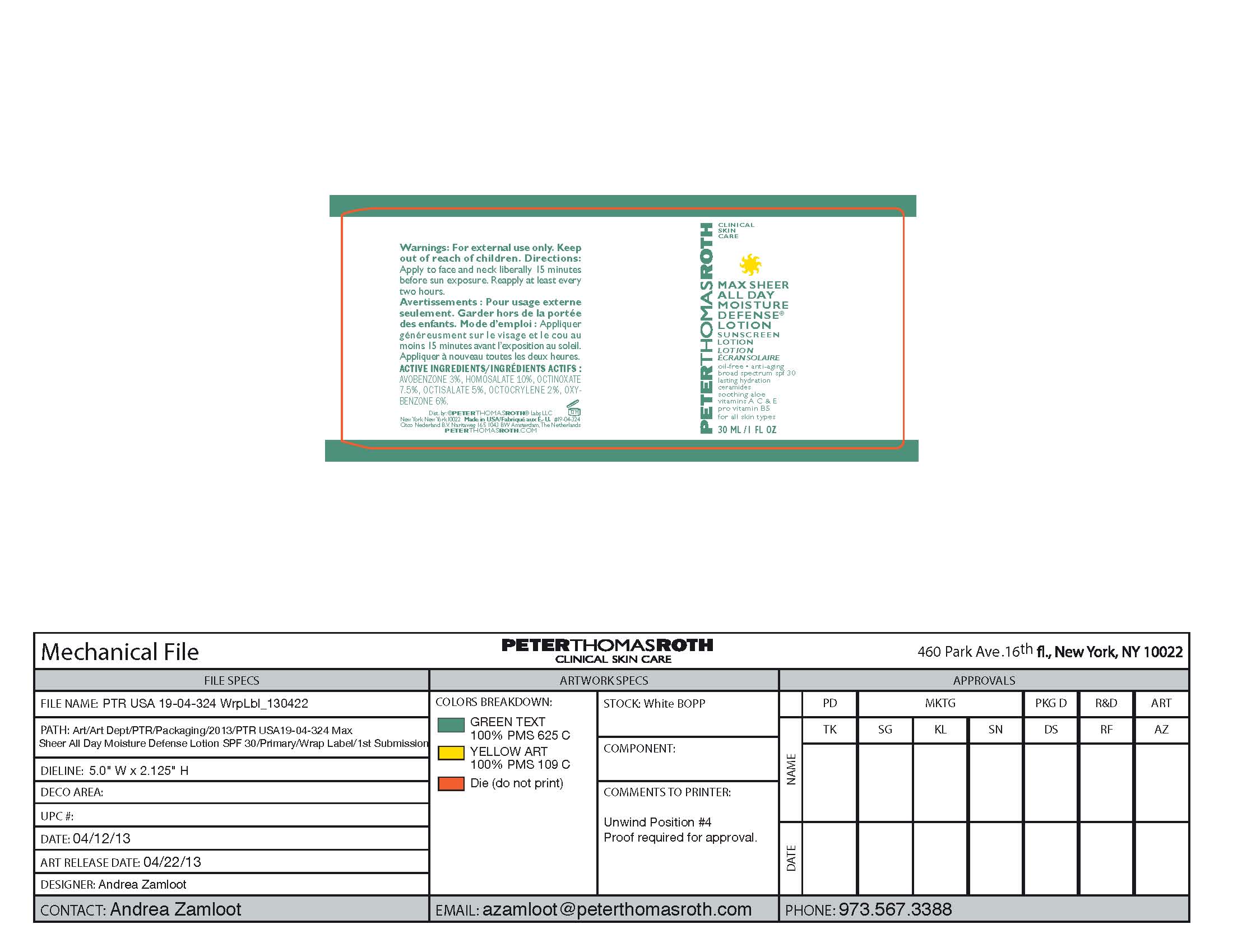
Label: MAX SHEER ALL DAY MOISTURE DEFENSE- sunscreen lotion spf 30 lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 54031-324-00, 54031-324-01, 54031-324-03, 54031-324-04, view more54031-324-05, 54031-324-06 - Packager: Peter Thomas Roth Labs LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 28, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Warnings
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if involved in swimming or intense physical activities where sweating will occur
- reapply at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
Allantoin, Aloe Barbadensis Leaf Juice, Aluminum Starch Octenylsuccinate, Ascorbyl Palmitate, Bentonite, Butylene Glycol, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Carbomer, Ceramide 3, Cetyl Alcohol, Dimethicone, Ginkgo Biloba Leaf Extract, Glyceryl Stearate, Panthenol, PEG-100 Stearate, Phenoxyethanol, Phospholipids, Potassium Sorbate, Propylene Glycol, Retinyl Palmitate, Sodium Benzoate, Stearic Acid, Tetrasodium EDTA, Tocopheryl Acetate, Triethanolamine, Water/Aqua/Eau.
- Other Information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MAX SHEER ALL DAY MOISTURE DEFENSE
sunscreen lotion spf 30 lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54031-324 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE .001 g in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE .0008 g in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE .0006 g in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE .0005 g in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE .0003 g in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE .0002 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 0.0048 g in 1 mL Propylene Glycol (UNII: 6DC9Q167V3) 0.0004 g in 1 mL GLYCERYL 1-STEARATE (UNII: 258491E1RZ) 0.0003 g in 1 mL PEG-100 Stearate (UNII: YD01N1999R) 0.00025 g in 1 mL ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) 0.0003 g in 1 mL MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) 0.0002 g in 1 mL Palmitic Acid (UNII: 2V16EO95H1) 0.0002 g in 1 mL Phenoxyethanol (UNII: HIE492ZZ3T) 0.0001 g in 1 mL Stearic Acid (UNII: 4ELV7Z65AP) 0.0001 g in 1 mL TROLAMINE (UNII: 9O3K93S3TK) 0.0001 g in 1 mL Bentonite (UNII: A3N5ZCN45C) 0.000025 g in 1 mL CARBOMER 940 (UNII: 4Q93RCW27E) 0.000025 g in 1 mL Dimethicone (UNII: 92RU3N3Y1O) 0.000025 g in 1 mL Allantoin (UNII: 344S277G0Z) 0.00002 g in 1 mL PEG-8 Dimethicone (UNII: GIA7T764OD) 0.000014 g in 1 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.00001 g in 1 mL Cetyl Alcohol (UNII: 936JST6JCN) 0.00001 g in 1 mL Panthenol (UNII: WV9CM0O67Z) 0.00001 g in 1 mL Aluminum Starch Octenylsuccinate (UNII: I9PJ0O6294) 0.0000087 g in 1 mL EDETATE SODIUM (UNII: MP1J8420LU) 0.0000084 g in 1 mL Myristic Acid (UNII: 0I3V7S25AW) 0.000006 g in 1 mL Potassium Sorbate (UNII: 1VPU26JZZ4) 0.0000025 g in 1 mL Sodium Benzoate (UNII: OJ245FE5EU) 0.0000025 g in 1 mL Arachidic Acid (UNII: PQB8MJD4RB) 0.000002 g in 1 mL Lauric Acid (UNII: 1160N9NU9U) 0.000002 g in 1 mL Ascorbic Acid (UNII: PQ6CK8PD0R) 0.000001 g in 1 mL Octyldodecanol (UNII: 461N1O614Y) 0.000001 g in 1 mL VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) 0.000001 g in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 0.000001 g in 1 mL Sodium Propoxyhydroxypropyl Thiosulfate Silica (UNII: 208G222332) 0.000001 g in 1 mL .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 0.000001 g in 1 mL Ceramide NP (UNII: 4370DF050B) 0.00000099 g in 1 mL Butylene Glycol (UNII: 3XUS85K0RA) 0.000000395 g in 1 mL GINKGO (UNII: 19FUJ2C58T) 0.0000002 g in 1 mL Butyl Acetate (UNII: 464P5N1905) 0.000000001 g in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54031-324-01 1 in 1 CARTON 07/29/2013 1 NDC:54031-324-00 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:54031-324-03 15 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/29/2013 3 NDC:54031-324-04 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/29/2013 4 NDC:54031-324-06 1 in 1 CARTON 07/29/2013 4 NDC:54031-324-05 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/29/2013 Labeler - Peter Thomas Roth Labs LLC (780458944) Registrant - Peter Thomas Roth Labs LLC (780458944) Establishment Name Address ID/FEI Business Operations June Jacobs Labs LLC 082439410 manufacture(54031-324)