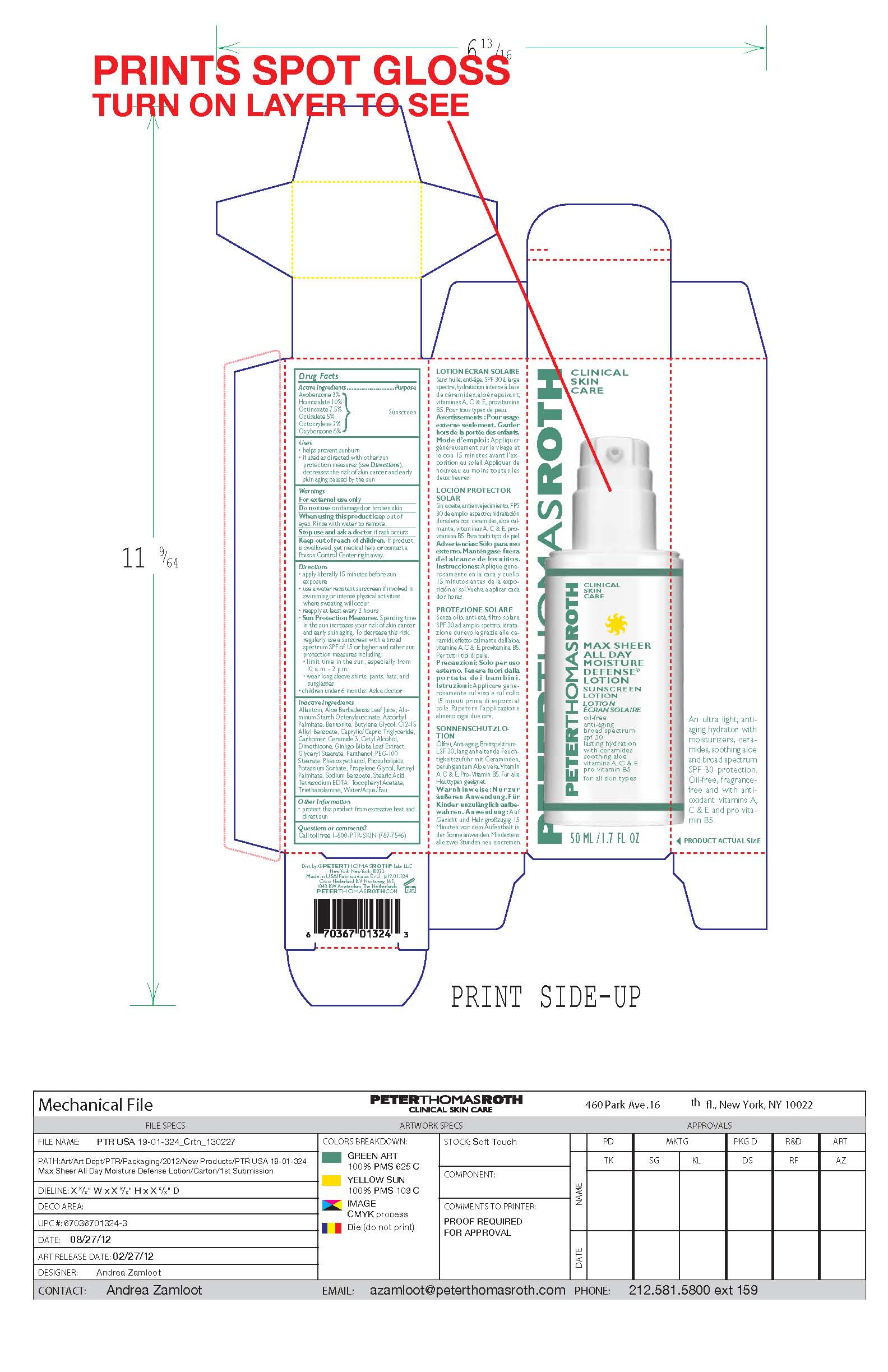
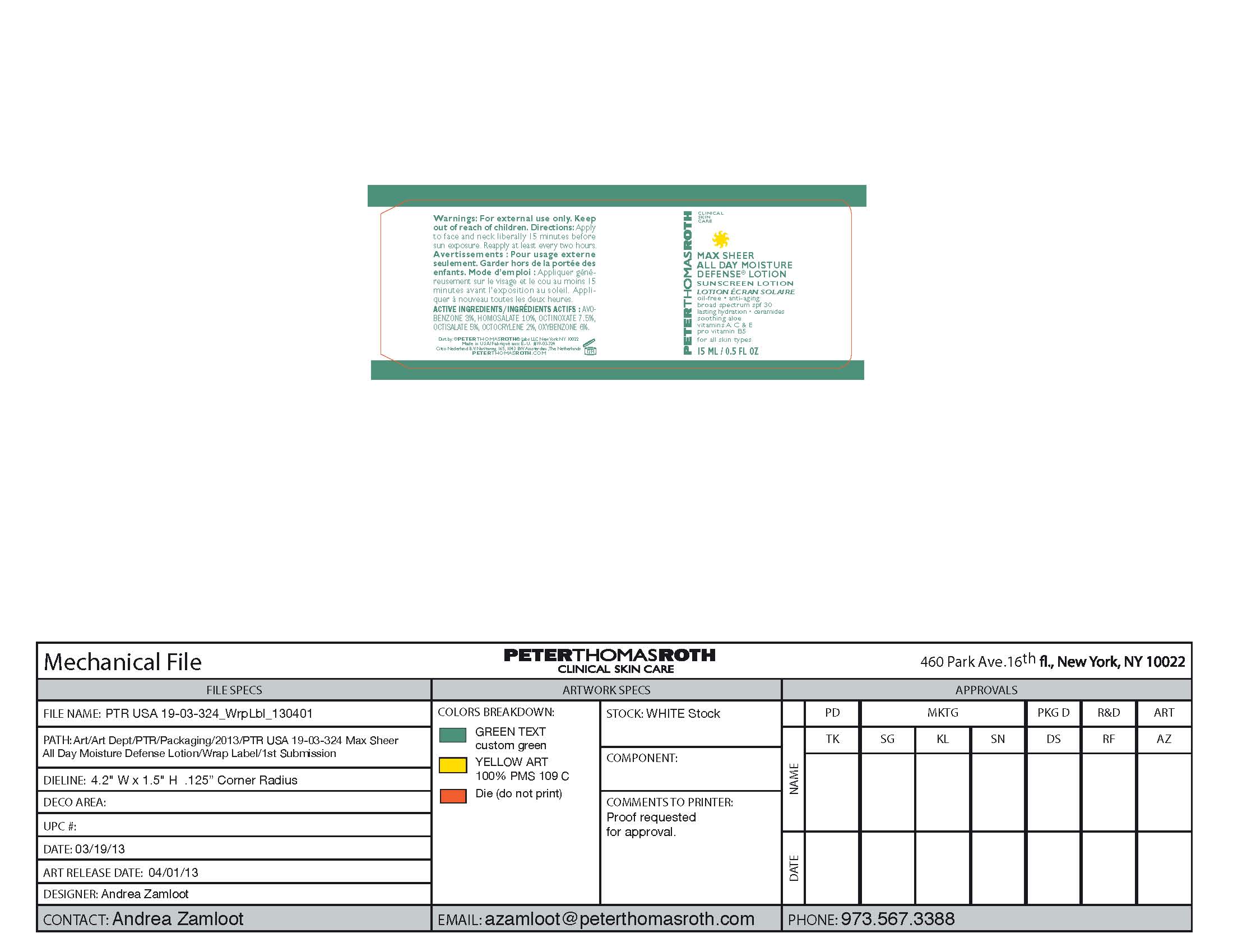
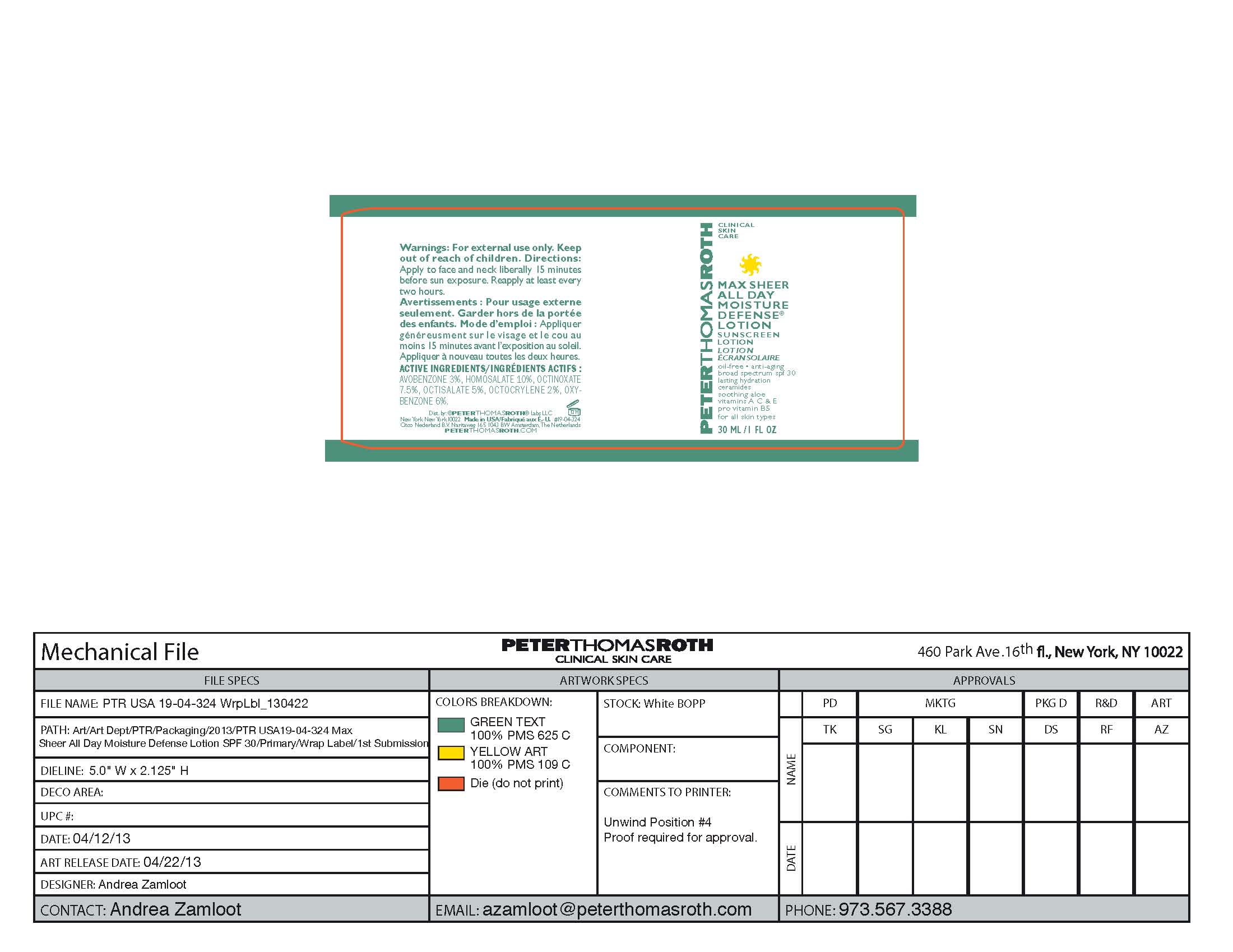
Drug Facts
Active Ingredients
Avobenzone 3%
Homosalate 10%
Octinoxate 7.5%
Octisalate 5%
Octocrylene 2%
Oxybenzone 6%
Purpose
Sunscreen
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Keep out of reach of children.
- If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if involved in swimming or intense physical activities where sweating will occur
- reapply at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
Allantoin, Aloe Barbadensis Leaf Juice, Aluminum Starch Octenylsuccinate, Ascorbyl Palmitate, Bentonite, Butylene Glycol, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Carbomer, Ceramide 3, Cetyl Alcohol, Dimethicone, Ginkgo Biloba Leaf Extract, Glyceryl Stearate, Panthenol, PEG-100 Stearate, Phenoxyethanol, Phospholipids, Potassium Sorbate, Propylene Glycol, Retinyl Palmitate, Sodium Benzoate, Stearic Acid, Tetrasodium EDTA, Tocopheryl Acetate, Triethanolamine, Water/Aqua/Eau.