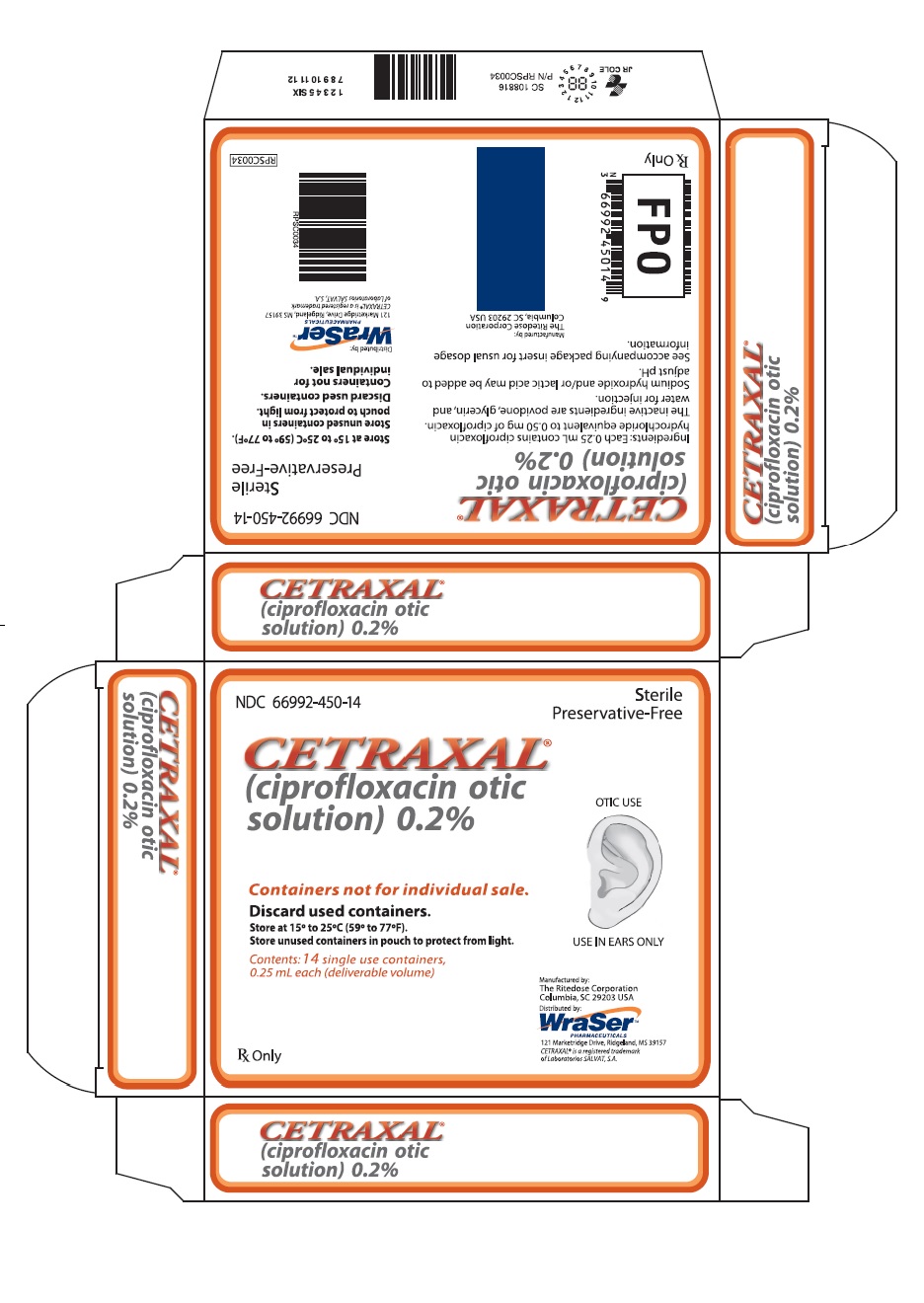
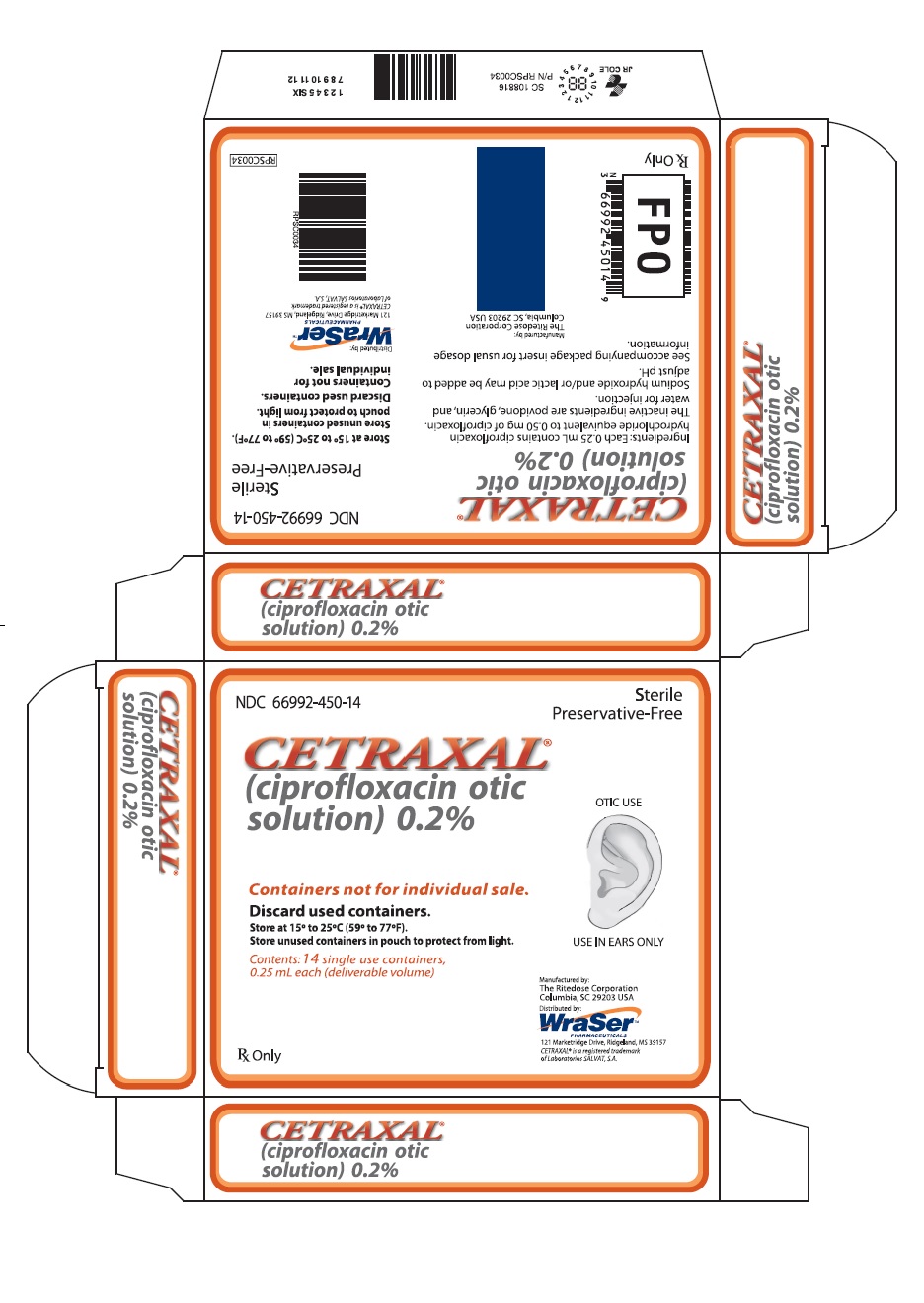
Label: CETRAXAL- ciprofloxacin solution/ drops
- NDC Code(s): 66992-450-14
- Packager: Wraser Llc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Cetraxal safely and effectively. See full prescribing information for Cetraxal.
Cetraxal (Ciprofloxacin otic solution) 0.2%
Initial U.S. Approval: 1987INDICATIONS AND USAGE
CETRAXAL is a quinolone antimicrobial indicated for the treatment of acute otitis externa due to susceptible isolates of Pseudomonas aeruginosaor Staphylococcus aureus. (1)
DOSAGE AND ADMINISTRATION
Contents of one single use container should be instilled into the affected ear twice daily (approximately 12 hours apart) for 7 days. (2)
DOSAGE FORMS AND STRENGTHS
CETRAXAL is a sterile, preservative-free otic solution of ciprofloxacin hydrochloride equivalent to 0.2 % ciprofloxacin (0.5 mg in 0.25 mL) in each single use container. (3)
CONTRAINDICATIONS
History of hypersensitivity to ciprofloxacin. (4)
WARNINGS AND PRECAUTIONS
- CETRAXAL is for otic use only. (5.1)
- Hypersensitivity: discontinue at the first appearance of a skin rash or any other sign of hypersensitivity. (5.2)
- Use of CETRAXAL may result in overgrowth of nonsusceptible organisms. (5.3)
ADVERSE REACTIONS
The most common adverse reactions reported in 2-3% of patients treated with CETRAXAL were application site pain, ear pruritus, fungal ear superinfection and headache. (6)
To report SUSPECTED ADVERSE REACTIONS, contact WraSer Pharmaceuticals at 1-888-252-3901or FDA at 1-800-FDA-1088or www.fda.go/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Otic Use Only
5.2 Hypersensitivity
5.3 Growth of Resistant Organisms with Prolonged Use
5.4 Lack of Clinical Response
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Directions for Use
17.2 Hypersensitivity
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The contents of one single use container (deliverable volume: 0.25 mL) should be instilled into the affected ear twice daily (approximately 12 hours apart) for 7 days.
Wash hands before use. The solution should be warmed, by holding the container in the hands for at least 1 minute, to minimize the dizziness that may result from the instillation of a cold solution into the ear canal. The patient should lie with the affected ear upward and then the solution should be instilled. This position should be maintained for at least 1 minute to facilitate penetration of the drops into the ear. Repeat, if necessary, for the opposite ear.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Otic Use Only
CETRAXAL is for otic use only. It should not be used for injection, for inhalation or for topical ophthalmic use.
5.2 Hypersensitivity
CETRAXAL should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity.
-
6 ADVERSE REACTIONS
Because clinical studies are conducted under widely varying conditions, adverse drug reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
In a randomized, active-controlled clinical trial, approximately 300 patients with clinical signs and symptoms of otitis externa were treated with CETRAXAL. The most frequently reported adverse reactions were application site pain, ear pruritus, fungal ear superinfection, and headache, each reported in approximately 2-3% of patients.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
Reproduction studies have been performed in rats and mice using oral doses of up to 100 mg/kg and intravenous (IV) doses up to 30 mg/kg and have revealed no evidence of harm to the fetus as a result of ciprofloxacin. In rabbits, ciprofloxacin (30 and 100 mg/kg orally) produced gastrointestinal disturbances resulting in maternal weight loss and an increased incidence of abortion, but no teratogenicity was observed at either dose. After intravenous administration of doses up to 20 mg/kg, no maternal toxicity was produced in the rabbit, and no embryotoxicity or teratogenicity was observed.
Animal reproduction studies have not been conducted with CETRAXAL. No adequate and well-controlled studies have been performed in pregnant women. Caution should be exercised when CETRAXAL is used by a pregnant woman.
8.3 Nursing Mothers
Ciprofloxacin is excreted in human milk with systemic use. It is not known whether ciprofloxacin is excreted in human milk following otic use. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of CETRAXAL in infants below one year of age have not been established. The efficacy of CETRAXAL in treating otitis externa in pediatric patients one year or older has been demonstrated in controlled clinical trials (see Section14 Clinical Studies).
There is no evidence that the otic administration of quinolones has any effect on weight bearing joints, even though systemic administration of some quinolones has been shown to cause arthropathy in immature animals.
-
11 DESCRIPTION
CETRAXAL (ciprofloxacin otic solution) 0.2% contains the synthetic antimicrobial agent ciprofloxacin hydrochloride. CETRAXAL is a sterile, preservative-free solution for otic use. Each single use container of CETRAXAL delivers 0.25 mL of solution equivalent to 0.5 mg of ciprofloxacin. The inactive ingredients are povidone, glycerin, and water for injection. Sodium hydroxide and/or lactic acid may be added to adjust pH.
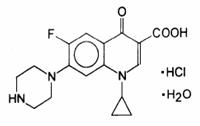
Ciprofloxacin, a fluroquinolone is available as the monohydrochloride, monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its molecular formula is C 17H 18FN 3O 3•HCl•H 2O, and molecular weight is 385.82.
The chemical structure of ciprofloxacin hydrochloride is:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ciprofloxacin is a fluoroquinolone antimicrobial (see 12.4 Clinical Pharmacology, Microbiology).
12.3 Pharmacokinetics
The plasma concentrations of ciprofloxacin were not measured following administration of 0.25 mL CETRAXAL (total dose: 0.5 mg ciprofloxacin). However, the maximum plasma concentration of ciprofloxacin is anticipated to be less than 5 ng/mL.
12.4 Microbiology
The bactericidal action of ciprofloxacin results from interference with the enzyme DNA gyrase, which is needed for the synthesis of bacterial DNA.
Bacterial resistance to quinolones can develop through chromosomally- or plasmid-mediated mechanisms.
The mechanism of action of fluoroquinolones, including ciprofloxacin, is different from that of macrolides. Therefore, ciprofloxacin may be active against pathogens that are resistant to these antibiotics, and these antibiotics may be active against pathogens that are resistant to ciprofloxacin. In vitrostudies demonstrated cross-resistance between ciprofloxacin and some fluoroquinolones.
Ciprofloxacin has been shown to be active against most isolates of the following bacteria, both in vitroand in clinical infections of acute otitis externa as described in Section 1 Indications and Usage.
Staphylococcus aureus
Pseudomonas aeruginosa. -
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term carcinogenicity studies in mice and rats have been completed for ciprofloxacin. After daily oral doses of 750 mg/kg (mice) and 250 mg/kg (rats) were administered for up to 2 years, there was no evidence that ciprofloxacin had any carcinogenic or tumorigenic effects in these species. No long-term studies of CETRAXAL have been performed to evaluate carcinogenic potential.
Eight in vitromutagenicity tests have been conducted with ciprofloxacin, and the test results are listed below:
- Salmonella/Microsome Test (Negative)
- Escherichia coliDNA Repair Assay (Negative)
- Mouse Lymphoma Cell Forward Mutation Assay (Positive)
- Chinese Hamster V79 Cell HGPRT Test (Negative)
- Syrian Hamster Embryo Cell Transformation Assay (Negative)
- Saccharomyces cerevisiaePoint Mutation Assay (Negative)
- Saccharomyces cerevisiaeMitotic Crossover and Gene Conversion Assay (Negative)
- Rat Hepatocyte DNA Repair Assay (Positive).
Two of the 8 in vitrotests were positive, but results of the following 3 in vivotest systems gave negative results:
- Rat Hepatocyte DNA Repair Assay
- Micronucleus Test (Mice)
- Dominant Lethal Test (Mice).
Fertility studies performed in rats at oral doses of ciprofloxacin up to 100 mg/kg/day revealed no evidence of impairment. This would be over 100 times the maximum recommended clinical dose of ototopical ciprofloxacin based upon body surface area, assuming total absorption of ciprofloxacin from the ear of a patient treated with CETRAXAL twice per day.
-
14 CLINICAL STUDIES
In a randomized, multi-center, evaluator-blinded study of patients with acute otitis externa, patients were treated with either CETRAXAL twice daily or neomycin and polymyxin B sulfates and hydrocortisone otic solution (PNH) three times daily for 7 days.
In the per protocol population, clinical cure was achieved at the end of a 7-day treatment in 70% (173/247) for the CETRAXAL treated group versus 60% (147/243) for the control treated group.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CETRAXAL is a clear, colorless, sterile, preservative-free solution. CETRAXAL is supplied as a 0.2% otic solution in a low-density polyethylene (LDPE) single use container. Each single use container delivers 0.25 mL of solution equivalent to 0.5 mg of ciprofloxacin; 14 single use containers are packaged in a foil overwrap pouch in a carton (NDC 66992-450-14).
-
17 PATIENT COUNSELING INFORMATION
17.1 Directions for Use
Patients should be advised that CETRAXAL is for otic use only. It is not for ophthalmic or inhalation use. It is not for injection.
CETRAXAL should be given 2 times each day (about 12 hours apart) in each infected ear.
CETRAXAL should be used for as long as it is prescribed, even if the symptoms improve. The patient should be advised to follow these directions while on CETRAXAL:
- Wash their hands before use.
- Warm the container in their hands for at least one minute prior to use to minimize dizziness that may result from the instillation of a cold solution into the ear canal. Twist off and discard top of container.
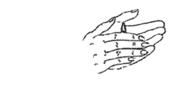
- Lie with the affected ear upward and then instill the contents of one container into the ear. Maintain this position for at least one minute to facilitate penetration of the drops into the ear.

- Repeat, if necessary, for the opposite ear.
- Discard used container.
- Store unused containers in pouch to protect from light.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CETRAXAL
ciprofloxacin solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66992-450 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CIPROFLOXACIN HYDROCHLORIDE (UNII: 4BA73M5E37) (CIPROFLOXACIN - UNII:5E8K9I0O4U) CIPROFLOXACIN 0.5 mg in 0.25 mL Inactive Ingredients Ingredient Name Strength POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66992-450-14 14 in 1 CARTON 05/14/2009 1 0.25 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021918 05/14/2009 Labeler - Wraser Llc (121828334)