Label: COLGATE SENSITIVE PLUS WHITENING- potassium nitrate and sodium fluoride paste, dentifrice
- NDC Code(s): 35000-023-34, 35000-023-46, 35000-023-60
- Packager: Colgate-Palmolive Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
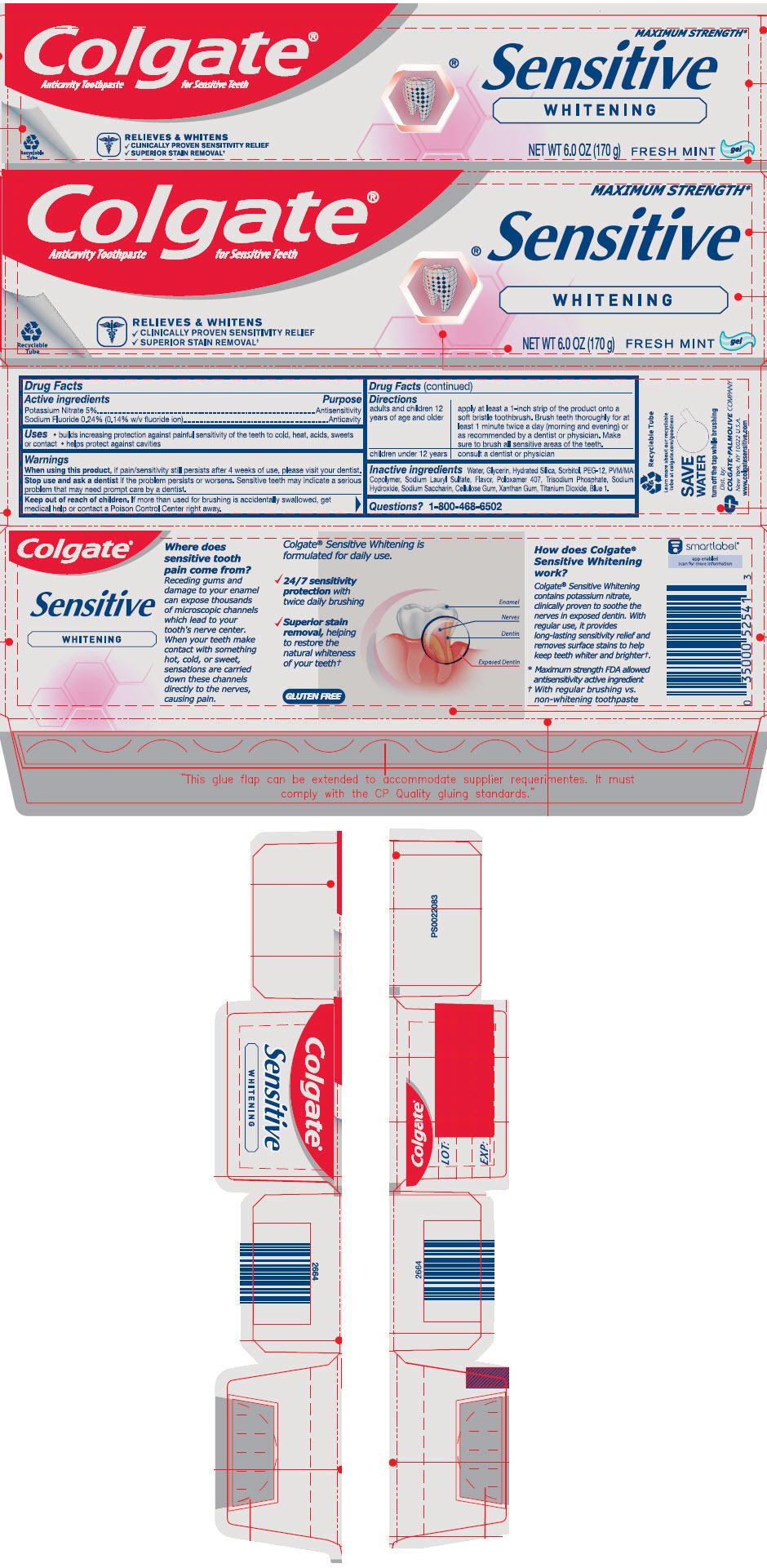
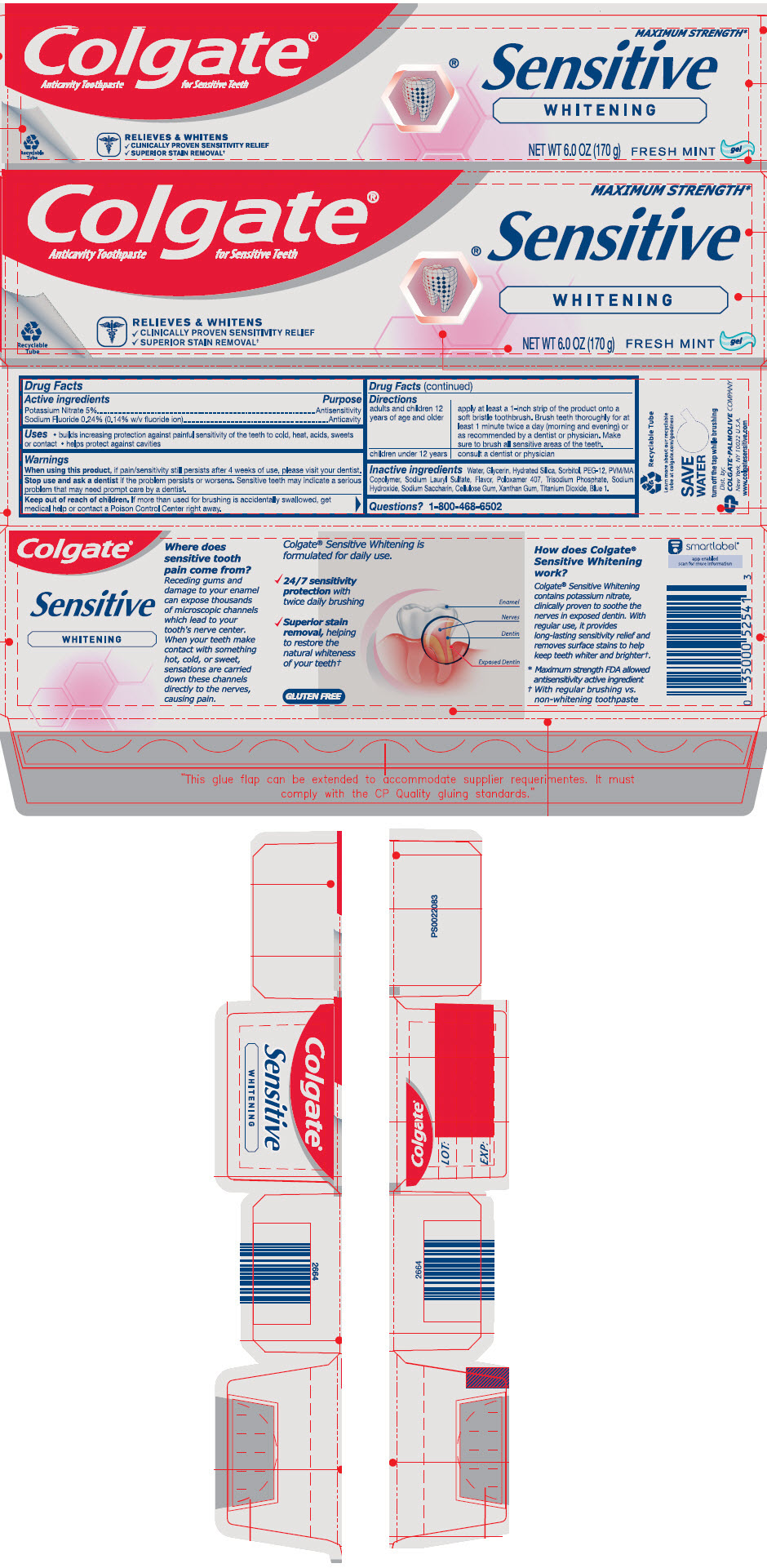
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
adults and children 12 years of age and older apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist or physician. Make sure to brush all sensitive areas of the teeth. children under 12 years consult a dentist or physician - Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 170 g Tube Carton
-
INGREDIENTS AND APPEARANCE
COLGATE SENSITIVE PLUS WHITENING
potassium nitrate and sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35000-023 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM NITRATE (UNII: RU45X2JN0Z) (NITRATE ION - UNII:T93E9Y2844) POTASSIUM NITRATE 50 mg in 1 g SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.1 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) HYDRATED SILICA (UNII: Y6O7T4G8P9) SORBITOL (UNII: 506T60A25R) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) METHYL VINYL ETHER AND MALEIC ACID COPOLYMER (1750000 WAMW) (UNII: 9F20VSM0VU) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLOXAMER 407 (UNII: TUF2IVW3M2) SODIUM PHOSPHATE, TRIBASIC, DODECAHYDRATE (UNII: B70850QPHR) SODIUM HYDROXIDE (UNII: 55X04QC32I) SACCHARIN SODIUM (UNII: SB8ZUX40TY) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) XANTHAN GUM (UNII: TTV12P4NEE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BLUE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35000-023-60 1 in 1 CARTON 02/25/2019 1 170 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:35000-023-34 2 in 1 CARTON 02/25/2019 2 170 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:35000-023-46 1 in 1 CARTON 06/15/2020 3 130 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M022 02/25/2019 Labeler - Colgate-Palmolive Company (001344381)